


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
أقرأ أيضاً
التاريخ: 2-11-2019
التاريخ: 11-7-2018
التاريخ: 31-12-2021
التاريخ: 3-11-2019
|
Absorption of infrared radiation causes transitions between vibrational energy states of a molecule. A simple diatomic molecule, such as H−Cl, has only one vibrational mode available to it, a stretching vibration somewhat like balls on the ends of a spring:
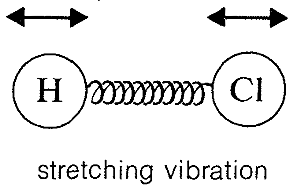
Molecules with three or more atoms can vibrate by stretching and also by bending of the chemical bonds, as indicated below for carbon dioxide:
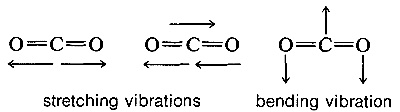
The absorption frequencies in the infrared spectra of molecules correspond to changes in the stretching or bending vibrations or both. In general, a polyatomic molecule with nn atoms will have 3n−6 modes of vibration of which n−1 are stretching vibrations and 2n−5 are bending vibrations. There are circumstances, however, where fewer vibrational modes are possible. If the molecule is linear, like CO2, then there are 3n−5 possible vibrations, and some of these vibrations may be equivalent (degenerate vibrations in the language of spectroscopists). For example, CO2 should have 3n−5 or vibrational modes, two of which are stretching and two of which are bending modes. However, the two bending modes are equivalent because the direction in which the molecule bends is immaterial; in-plane or out-of-plane bending are the same:
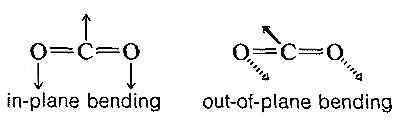
Diatomic molecules such as HCl have one vibrational mode, but it is important to note that symmetrical diatomic molecules, such as O2, N2, Cl2, F2, and H2, do not absorb in the infrared region of the spectrum. This is because absorption cannot occur if the vibration is electrically symmetrical. Fortunately, then, the infrared spectra can be recorded in air because the main components of air, N2 and O2, do not interfere.
In practice, infrared spectra can be obtained with gaseous, liquid, or solid samples. The sample containers (cells) and the optical parts of the instrument are made of rock salt (NaCl) or similar material that transmits infrared radiation (glass is opaque).
Typical infrared spectra are shown in Figure 9-9 for 2-propanone (acetone), CH3−CO−CH3, and 2-butanone (methyl ethyl ketone), CH3−CO−CH2−CH3. In accord with current practice, the position of absorption (horizontal scale) is recorded in units of wave numbers (ν¯, cm−1). The vertical scale measures the intensity of radiation transmitted through the sample. Zero transmission means complete absorption of radiation by the sample as at 1740cm−1 in Figure 9-9. The other absorption bands in Figure 9-9 that correspond to excitation of stretching or bending vibrations are not as intense as the absorption at 1740cm−1.
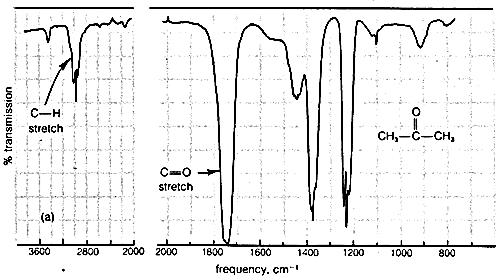
Figure 9-9: Infrared absorption spectra of (a) 2-propanone and (b) 2-butanone in the vapor phase.



|
|
|
|
"عادة ليلية" قد تكون المفتاح للوقاية من الخرف
|
|
|
|
|
|
|
ممتص الصدمات: طريقة عمله وأهميته وأبرز علامات تلفه
|
|
|
|
|
|
|
المجمع العلمي للقرآن الكريم يقيم جلسة حوارية لطلبة جامعة الكوفة
|
|
|