


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
أقرأ أيضاً
التاريخ: 7-1-2022
التاريخ: 2024-04-24
التاريخ: 14-1-2022
التاريخ: 9-3-2018
|
Many separation methods are based on chromatography, that is, separation of the components of a mixture by differences in the way they become distributed (or partitioned) between two different phases. To illustrate with an extreme example, suppose we have a mixture of gaseous methane and ammonia and contact this mixture with water. Ammonia, being very soluble in water (~90g per 100g water at 1atm pressure), will mostly go into the water phase, whereas the methane, being almost insoluble (~0.003g per 100g of water) will essentially remain entirely in the gas phase. Such a separation of methane and ammonia would be a one-stage partitioning between gas and liquid phases and, clearly, could be made much more efficient by contacting the gas layer repeatedly with fresh water. Carried through many separate operations, this partitioning procedure is, at best, a tedious process, especially if the compounds to be separated are similar in their distributions between the phases. However, partitioning can be achieved nearly automatically by using chromatographic columns, which permit a stationary phase to be contacted by a moving phase. To illustrate, suppose a sample of a gaseous mixture of ammonia and methane is injected into a long tube (column) filled with glass beads moistened with water (the stationary phase), and a slow stream of an inert carrier gas, such as nitrogen or helium, is passed in to push the other gases through. A multistage partitioning would occur as the ammonia dissolves in the water and the resulting gas stream encounters fresh water as it moves along the column. Carrier gas enriched with methane would emerge first and effluent gas containing ammonia would come out later. This is a crude description of the method of gas-liquid chromatography (abbreviated often as glc, GC, or called vapor-phase chromatography, vpc). This technique has become so efficient as to revolutionize the analysis and separation of almost any organic substance that has even a slight degree of volatility at some reasonably attainable temperature. The most modern glc equipment runs wholly under computer control, with preprogrammed temperatures and digital integration of the detector output. A wide variety of schemes is available for measuring the concentration of materials in the effluent carrier gas, and some of these are of such extraordinary sensitivity that only very small samples are necessary (10−9g or less).
Figure 9-1: Schematic diagram of a gas-liquid chromatography apparatus. The detector is arranged to measure the difference in some property of the carrier gas alone versus the carrier gas plus effluent sample at the exit. Differences In thermal conductivity are particularly easy to measure and give reasonably high detection sensitivities.
In the usual glc procedure, a few microliters of an organic liquid to be analyzed are injected into a vaporizer and carried with a stream of gas (usually helium) into a long heated column that is packed with a porous solid (such as crushed firebrick) impregnated with a nonvolatile liquid. Gas-liquid partitioning occurs, and small differences between partitioning of the components can be magnified by the large number of repetitive partitions possible in a long column. Detection often is achieved simply by measuring changes in thermal conductivity of the effluent gases. A schematic diagram of the apparatus and a typical separation pattern are shown in Figures 9-1 and 9-2. The method is extraordinarily useful for detection of minute amounts of impurities provided these are separated from the main peak. Glc also can be used effectively to purify materials as well as to detect impurities. To do this, the sample size and the size of the apparatus may be increased, or an automatic system may be used wherein the products from many small-scale runs are combined.
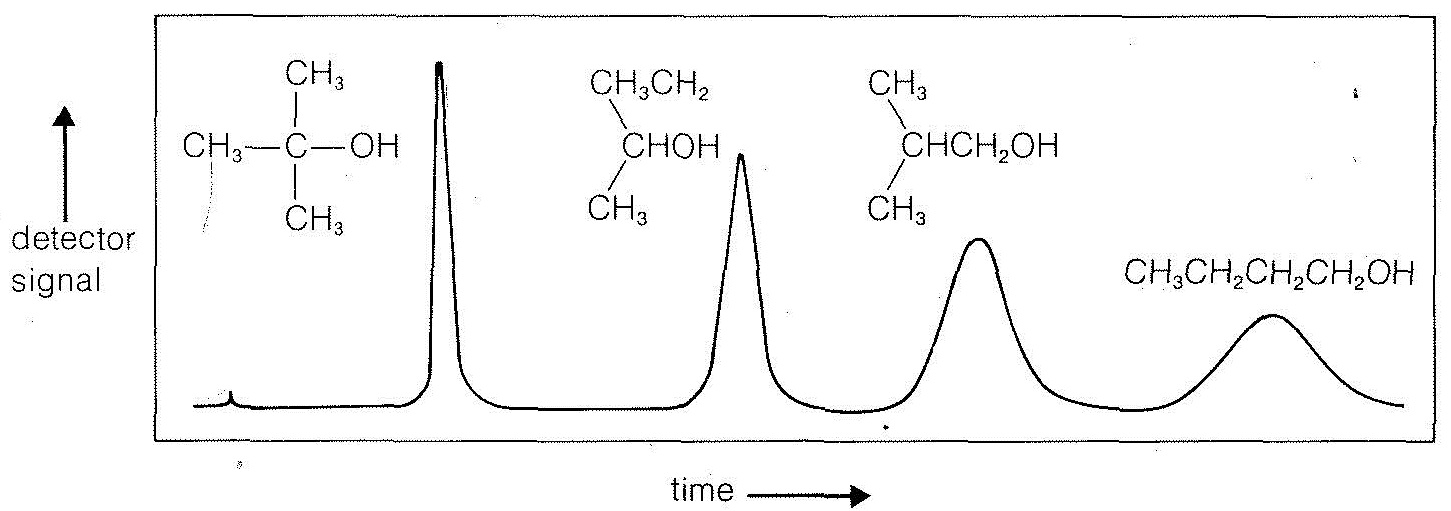
Figure 9-2: A gas-liquid chromatogram of a mixture of the isomeric butanols at constant column temperature. A tiny peak on the far left is a trace of air injected with the sample. The retention times of the various isomers are in the same order as the boiling points, which are, from left to right, 82o82o, 99.5o99.5o, 108o108o, and 117o117o. The areas under each peak correspond to the relative amounts of material present. Raising the column temperature at a preprogrammed rate while developing the chromatogram speeds up the removal of the slower-moving components and sharpens their peaks. Also, by diversion of the gas stream to appropriate cold traps it is possible to collect pure fractions of each component.I



|
|
|
|
"عادة ليلية" قد تكون المفتاح للوقاية من الخرف
|
|
|
|
|
|
|
ممتص الصدمات: طريقة عمله وأهميته وأبرز علامات تلفه
|
|
|
|
|
|
|
المجمع العلمي للقرآن الكريم يقيم جلسة حوارية لطلبة جامعة الكوفة
|
|
|