

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
More on Interelectronic Repulsion and Bond Angles
المؤلف:
John D. Roberts and Marjorie C. Caserio
المصدر:
Basic Principles of Organic Chemistry : LibreTexts project
الجزء والصفحة:
........
31-12-2021
2477
More on Interelectronic Repulsion and Bond Angles
Molecules of the type AX4, which have four identical ligands on the central atom and no unshared electrons on A (e.g., CH4 and CCl4), are expected to be, and are, tetrahedral. By the same reasoning, three electron pairs around one atom should seek a planar arrangement with 120o angles to minimize electron repulsion; accordingly, species of the type AX3, which have no unshared pairs on A (e.g., BF3 and CH3⊕), have this geometry. With only two electron pairs, the preferred arrangement is linear.
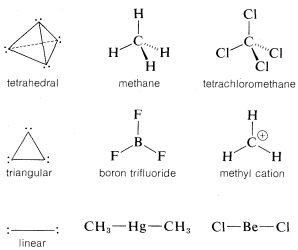
The bond angles of compounds with multiple bonds can be explained similarly. For example, in ethene the four electrons of the double bond occupy the region in space between the two carbon nuclei. The situation at either carbon is rather like the AX3 case, except that one of the ligands now has a double complement of bonding electrons:

Therefore the carbon orbitals are expected to be directed in one plane to give bond angles that deviate somewhat from 120o because of the high density of electrons in the multiple bond. Thus the H−C−H angle shrinks to 117o, whereas the H−C=C angles open up to 122o, because repulsion between electrons in the H−C=C bonds is greater than between electrons in the H−C−H bonds.
Electron-attracting power (or electronegativity) of the ligands also is important in determining bond angles. Thus for compounds of the type CH3X, in which X is a more electron-attracting group than carbon, the C−X bond is polarized in the sense H3Cδ⊕−−−Xδ⊖, and the carbon then should have some of the character of CH3⊕. Thus the H−C−H angles are expected to be greater than 109.5o, as in fact they are. In chloromethane, for example, the H−C−H angle is 111o.
Also, we can explain on the basis of electron repulsions why the bond angle in phosphine, :PH3 (93o), is less than that in ammonia, :NH3 (107.3o), and the bond angle in H:S¨⋅⋅:H (92.2o) is less than that in H:O¨⋅⋅ (104.5o). The important point is that phosphorus and sulfur are larger atoms than nitrogen and oxygen. This means than the H−S−H and H−P−H bond angles can be about 90o without bringing the hydrogens and the bonding pairs as close together as they are in H2O and NH3 where the bond angles are near to the tetrahedral value.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















