


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
أقرأ أيضاً
التاريخ: 26-8-2019
التاريخ: 2025-01-09
التاريخ: 20-10-2020
التاريخ: 16-9-2019
|
The most important type of stereoisomerism is that which arises when molecules possess two structures that are not identical and also are mirror images of one another. This is not a difficult or unfamiliar concept. Many things around us, such as our hands, and pairs of shoes, are not identical and also are mirror images of one another. In the same way, nonidentical molecules exist in which the only distinction between them is that one is the mirror image of the other. A common statement is that such isomers are mirror images of one another, but these images are not "superimposable." A simple example of this type of stereoisomerism is 2-chlorobutane,  , which can exist in two spatial configurations, 1 and 2, that correspond to reflections of each other. These isomers are specifically called enantiomers.
, which can exist in two spatial configurations, 1 and 2, that correspond to reflections of each other. These isomers are specifically called enantiomers.
Figure 5-3. If you put CH3 and CH3 together, and C2H5 and C2H5 together, you find that Cl and Cl are on opposite sides, and HH and HH are on opposite sides. No matter how you turn the models around, they cannot be superimposed unless you break bonds at the number 2 carbon and interchange the positions of any two atoms or groups.
Compounds that lack reflection symmetry - meaning that they are not identical with their mirror images - are said to be chiral (pronounced "ki-rall", rhymes with spiral). This term is derived from the Greek word χϵιρ= hand; and "handedness" or chirality is a property of dissymmetric molecules such that two configurational isomers are possible that are nonidentical mirror images. Compounds that possess reflection symmetry - meaning that they are identical with their mirror images - are said to be achiral. Enantiomers are not possible for achiral compounds. An enantiomeric pair is a pair of substances whose molecules are nonidentical mirror images.
The pressing question at this point is how can we tell whether a substances will be chiral or achiral. The most common origin of chirality in molecules, and the one originally recognized by van't Hoff and Le Bel, is the presence of one or more atoms, usually carbon atoms, each of which forms coplanar bonds to four different atoms or groups. This is the case for 2-chlorobutane, because the second tetrahedral carbon along the chain is bonded to four different groups: hydrogen, chlorine, methyl, and ethyl. Therefore there is a pair of enantiomers, 1 and 2. Another example is 2-bromo-2-chloro-1,1,1-trifluoroethane, which is a widely used inhalation anaesthetic. The four different groups in this case are hydrogen, chlorine, bromine, and trifluoromethyl; the pair of enantiomers is shown in Structures 3 and 4:

The atom that carries the four different substituents in 1 and 2, or 3 and 4, is called a chiral atoms or chiral center. The latter is the more general term because, as we shall see later , dissymmetry in molecules need not be centered at an atom.
Figure 5-3: Use of ball-and-stick models to show nonidentity of the enantiomers of 2-chlorobutane, 1 and 2
In evaluating a chemical structure for chirality, you should look for carbons carrying four different attached groups. There may be more than one chiral carbon, and you should be alert to the fact that structural differences in the attached groups do not necessarily show up at the first, or even the second, position along a chain. As an example, consider the chirality of 1,1,3-trimethylcyclohexane,

Carbons C2, C4, C5, and C6 are clearly achiral because each is connected to two identical groups, which for these atoms are hydrogens. The same is true for C1 because it is connected to two CH3 groups. You might conclude that C3 also is an achiral position because it is connected to two CH2 groups. But this would be wrong. If you look farther, you will see that the groups attached to C3 actually are different and are H, CH3, −CH2CH2CH2−, and −CH2C(CH3)2. Therefore 1,1,3-trimethylcyclohexane has a chiral center at C3. In contrast, the 1,1,4-isomer has no chiral centers because the groups attached to the ring at C4 are identical:
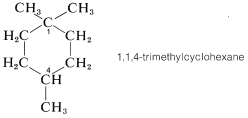
Several other terms that we shall use frequently in addition to chirality are racemic mixture, resolution, and racemization. A mixture of equal amounts of both enantiomers is a racemic mixture; separation of a racemic mixture into its component enantiomers is a resolution, and the conversion of either enantiomer into equal parts of both is called racemization.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|