


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
أقرأ أيضاً
التاريخ: 12-7-2018
التاريخ: 31-10-2016
التاريخ: 29-11-2019
التاريخ: 31-10-2016
|
A brilliant solution to the problem posed in the preceding section came in 1874 when J. H. van't Hoff proposed that all four valences of carbon are equivalent and directed to the corners of a regular tetrahedron. If we redraw the structures for C2H5Br as 1, we see that there is only one possible arrangement and, contrary to the impression we got from our earlier structural formulas, the bromine is equivalently located with respect to each of the hydrogens on the same carbon.
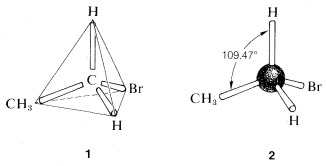
A convenient way of representing organic molecules in three dimensions, which shows the tetrahedral relationships of the atoms very clearly, uses the so-called ball-and-stick models. The sticks that represent the bonds or valences form the tetrahedral angles of 109.47o.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|