


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
أقرأ أيضاً
التاريخ: 22-12-2020
التاريخ: 18-12-2021
التاريخ: 22-12-2020
التاريخ: 19-12-2021
|
The idea that ethanoic acid (acetic acid) is a possible common starting material for the biosynthesis of many organic compounds was first proposed by Collie (1893) on purely structural grounds. He recognized a structural connection between a linear chain of recurring CH3CO units (a polyketomethylene chain, CH3COCH2COCH2COCH2CO−) and certain cyclic natural products. In the example given below, orsellinic acid is represented as if it were derived from a chain of four CH3COunits by a condensation-cyclization reaction:
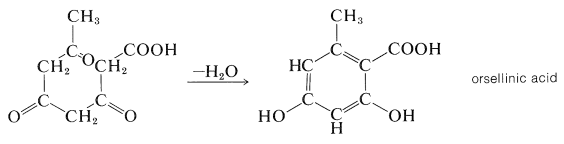
Experimental verification of Collie's hypothesis came many years later when isotopic hydrogen and carbon (H2, H3, C13, and C14 became available. Tracer studies showed that long-chain fatty acids are made by plants and animals from CH3CO units by successively linking together the carbonyl group of one to the methyl group of another (K. Bloch and F. Lynen, Nobel Prize, 1964). If ethanoic acid supplied to the organism is labeled at the carboxyl group with C14(C∗), the fatty acid has the label at alternate carbons:
Ethanoic acid is activated for biosynthesis by combination with the thiol, coenzyme A , Figure 18-7) to give the thioester, ethanoyl (acetyl) coenzyme A (CH3COSCoA). You may recall that the metabolic degradation of fats also involves this coenzyme and it is tempting to assume that fatty acid biosynthesis is simply the reverse of fatty acid metabolism to CH3COSCoA. However, this is not quite the case. In fact, it is a general observation in biochemistry that primary metabolites are synthesized by different routes from those by which they are metabolized .
A brief description of the main events in fatty-acid biosynthesis follows, and all of these steps must be understood to be under control of appropriate enzymes and their coenzymes even though they are omitted here.
The CH3CO group of ethanoyl coenzyme A is first transferred to a protein having a free thiol (SH) group to make another thioester, represented here as CH3COS−ACP, where ACP stands for Acyl-Carrier-Protein. The growing carbon chain remains bound to this protein throughout the synthesis:
CH3COSCoA+HS−ACP→CH3COS−ACP+CoASH (30.5.3)
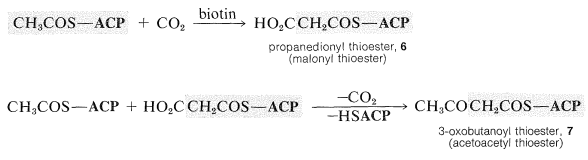
Carboxylation of CH3COS−ACP yields a propanedioyl thioester, 66, which then undergoes a Claisen condensation with a second mole of CH3COS−ACPaccompanied by decarboxylation to yield a 3-oxobutanoyl thioester, 7:
Reduction of the ketone group of the thioester (by NADPH) leads to a thiol ester of a four-carbon carboxylic acid. Repetitive condensations with thioester 66 followed by reduction eventually lead to fatty acids. Each repetition increases the chain length by two carbons:
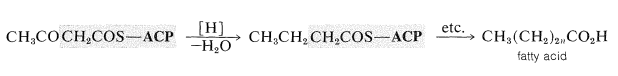
The preceding scheme is representative of fatty acid biosynthesis in plants, animals, and bacteria. The major difference is that plant and bacterial fatty acids usually contain more double bonds (or even triple bonds) than do animal fatty acids.



|
|
|
|
"عادة ليلية" قد تكون المفتاح للوقاية من الخرف
|
|
|
|
|
|
|
ممتص الصدمات: طريقة عمله وأهميته وأبرز علامات تلفه
|
|
|
|
|
|
|
المجمع العلمي للقرآن الكريم يقيم جلسة حوارية لطلبة جامعة الكوفة
|
|
|