


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
Read More
Date: 4-3-2021
Date: 2-1-2017
Date: 13-2-2021
|
Spin moment
The electron possesses intrinsic spin angular momentum with quantum number s = 1/2. There is an associated intrinsic magnetic moment, unrelated to any orbital motion, which can only adopt one of two discreet orientations relative to a magnetic field. The electron is really a point particle, with radius <10−20 m, much smaller than the classical radius, so the image of a spinning ball of charge in Fig. 1.1 is ultimately misleading. The mysterious built-in angular momentum emerges as a consequence of relativistic quantum mechanics. All fermions have spin and an associated magnetic moment. It turns out that the magnetic moment associated with the electron spin is not a half, but almost exactly one Bohr magneton. The gyromagnetic ratio γ is −(e/me) and the g-factor is close to 2.
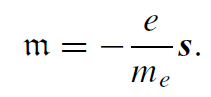
The spin magnetic magnetic quantum number is ms = ±1/2, so there are only the two possible angular momentum states. The component of spin along any axis is ±1/2h:

Spin angular momentum is therefore twice as efficient as orbital angular momentum at creating a magnetic moment. With higher-order corrections, g for the electron’s intrinsic spin moment turns out to be 2.0023. The spin moment of the electron is 1.00116 μB. For practical purposes, this small correction can be ignored.
The reality of the link between magnetism and angular momentum, known as the Einstein–de Haas effect, was demonstrated in an experiment carried out by John Stewart in 1917. A ferromagnetic rod is suspended from a torsion fibre so that it can turn about its own axis.Avertical magnetic field created by a solenoid is sufficient to overcome the demagnetizing field and saturate the magnetization of the ferromagnet. The current in the solenoid is then reversed, switching the direction of magnetization of the rod, thereby delivering an angular impulse due to the reversal of the angular momentum of the electrons. The impulse causes the rod to rotate, and from the angle of rotation and the torsion constant of the fibre it is possible to deduce the change of angular momentum. In the case of iron, forwhich the spontaneous magnetization Ms = 1710 kAm−1, the g-factor is found to be 2.09. This shows that the magnetization of iron is essentially due to electron spin. More surprising is the magnitude of the ferromagnetic moment, which works out at only 2.2μB per atom.1 The number of electrons per iron atom is equal to the atomic number, Z = 26, yet the ferromagnetic moment of iron corresponds to the spin moment of barely two of them. All the others form pairs with oppositely aligned spins, and contribute nothing.

Fig. 1.1: The Einstein–de Haas effect, demonstrating the relation between angular momentum and magnetic moment. A ferromagnetic rod is suspended on a fibre and the field in the solenoid is reversed, switching the direction of magnetization; the rod turns.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|