


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 8-1-2021
Date: 4-1-2021
Date: 8-1-2021
|
Multienzyme Complexes
Application of extracellular enzyme-based catalysis has mostly been concerned with single enzymes performing single-step reactions. Although there is still much work to be done with single enzyme biotransformations, other biotechnologists have focused attention on the next phase of extracellular biocatalysis, i.e. sequential multiple-step biotransformations as currently performed in biochemical pathways by cells and organelles. Multienzyme complexes are ordered assemblies of functionally and structurally different enzymes and proteins, which catalyse successive steps in a biochemical reaction pathway. Greater understanding of such complexes has provided scope to take enzyme-based catalysis beyond singlestep reaction processes to more sophisticated reaction schemes. A number of multienzyme structures have been studied and examples below reveal some of the opportunities and challenges that can be obtained from research on these complexes.
Apoptosomes
Individual cells have a capacity to self-terminate in a controlled manner, if required for the benefit of the organism as a whole, in a process called apopotosis.This process has an important function in processes that involve cell turnover such as development of an embryo, cell recycling, cell-based diseases and in removal or recycle of surplus, damaged or
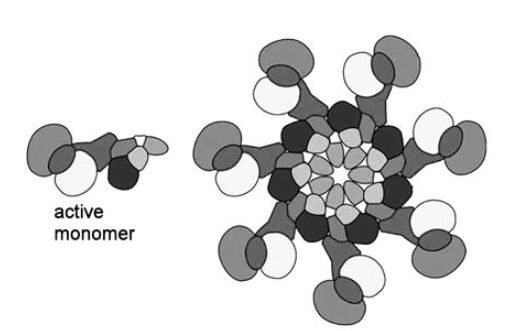
Figure 1 Apoptosome schematic showing the complex organisation of seven active monomers to form a wheel-like structure.
faulty cells. The process involves a suite of protease enzymes called caspases (cysteine, aspartic acid specific proteases).Normally these enzymes are in a dormant precursor state, but when activated they produce a cascade of proteolytic activity that destroys intracellular protein structures, enzymes and other vital proteins in an organised shutdown mechanism that results in cell death and subsequent removal of the dead cell by phagocytes.
Work on apoptosome structure is progressing and recent work reveals a model in which various protease and non-protease components are assembled in a wheel-like oligomeric structure (Figure 1).This model is based on an assembly of recombinant proteins because extraction of a functional apoptosome is still a major challenge for current isolation/extraction technology. The role of apoptosis in disease is also under investigation and it is clear that disturbance of the natural role of apoptosis can influence disease and dysfunction. In some diseases such as cancer, apoptosis would be antagonistic to cell proliferation and so it may be that oncogenes produce inhibitors that block normal apoptosis, whereas in degenerative diseases it may be a feature of this type of disease that premature activation of apoptosis is stimulated.There is clearly a great wealth of information waiting to be discovered both at the local level on how protease enzymes are orchestrated and at the higher cell and tissue level on how tissues are sculptured to meet the needs of the organism.
Proteosomes
These are large (typically 2000 kDa) multienzyme complexes consisting of more than 25 different proteins, which range in size from 22 to 110 kDa. They are found in both cytoplasm and nucleus of eukaryotic cells and structurally appear (side-on-view) as a cylindrical stack of four rings with six or seven proteins in each ring.14 The function of the proteosome is rapid hydrolysis of intracellular protein to enable fast turnover of protein. Proteins destined for hydrolysis by proteosomes are tagged by the regulatory protein ubiquitin and working together proteosomes & ubiquitin have a central role in the function of many critical cellular processes. Fast recycle of protein is important in many cellular processes such as embryogenesis, cell growth, cell differentiation, cell-cycle control, response to environmental stress and removal of damaged/faulty protein. It is also vital for continuous supply of amino acids for new protein synthesis and for immune responses, some key functions of the nervous system and acquisition of memory. The complex contains both endopeptidase (cut the inside of protein to produce large fragments) and exopeptidase (cut at the ends of polypeptide fragments to produce peptides and amino acids) and is therefore capable of complete hydrolysis of protein to amino acids. Proteosome also have a role in the presentation of antigens as part of the immune defence system and proteosome inhibitors have been investigated as possible tools to combat viral infections.
Cellulosomes
Cellulose is the most abundant source of carbon and chemical energy on Earth and around two-thirds of the billions of kilograms of industrial and domestic waste generated by individual countries around the world is potentially biodegradable because of the cellulose content. However, it is a very stable polymer of glucose, which is usually inter-mixed with other polysaccharides such as xylan and lignin to produce a complex structure that is resistant to standard mechanisms of chemical or biological degradation. Cellulolytic microorganisms have evolved a multifunctional multienzyme complex called a cellulosome, which provides a systematic hydrolysis of cellulose to release glucose sugars for growth and energy supply.The complexity of cellulose structure that plants have evolved to try to resist tissue loss often requires concerted cooperation between various cellulolytic wood-degrading microorganisms.
Typically, a cellulosome is composed of a central protein skeleton called scaffoldin on to which are attached link proteins called dockerins as depicted in Figure 2. Attached to dockerin proteins are various enzymes associated with cellulose hydrolysis and also attached are binding proteins that attach to the cellulose substrate and physically locate the complex on to cellulose. A raft of enzymes have been identified including endoglucanases, exoglucanases, b-glucosidase, xylanase,ligninase, pectinase and the amount and pattern of enzymes can be varied by a given microorganism to suit the different structured architectural forms of cellulose produced by a given plant.Flexibility and adaptability are key features that are highly desirable in biocatalysis and it is likely that there are some very clever principles that can be derived from detailed studies on the cellulosome.
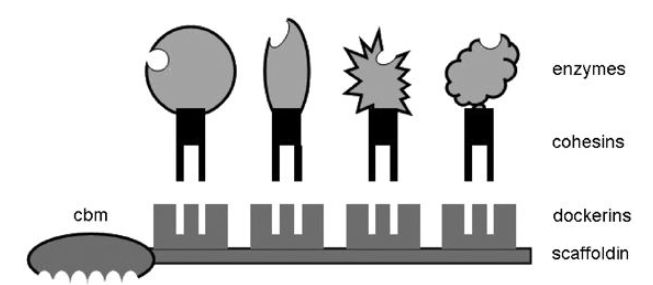
Figure 2 Cellulosome schematic showing various enzymes attached to cohesin proteins, which bind to dockerins attached to a central backbone called scaffoldin. Also attached to scaffoldin is a cellulose binding module (cbm).
Given an abundance of cellulose on Earth, it is not surprising that several thousand cellulolytic strains have been found among microorganisms. Recent work has
evaluated the potential of engineering cellulosomes for waste management by selecting particular enzyme components from different cellulolytic organisms for combination in a designer cellulosome for specified applications. Microorganisms have evolved cellulosomes to degrade cellulose for their own particular energy and growth requirements. It should be possible to extract the best set of catalytic features from the spectrum of different organisms and design cellulosomes that will reproduce the processes currently operating in landfill sites around the world by suites of cellulolytic organisms, but in a faster, more controlled process, that will also release the energy trapped in cellulose.
Multienzyme Complexes and Future Immobilisation Technology
An exciting prospect for biocatalysis is that studies on multienzyme complexes such as apoptosomes, proteosomes and cellulosomes will undoubtedly shape future methods of biocatalyst immobilisation technology. It may be possible to make immobilisation a more active participant in biotransformation reactions by introducing specificity to support materials via immobilised specific binding domains that improve accurate binding of particular substrate materials so increasing substrate specificity and catalytic power. It will be possible to mimic the cellulosome and immobilise an array of specific enzymes and regulatory proteins together for sophisticated multi-step biotransformations (Figure 2).
The future will provide one modular universal support material on to which can be attached any number of binding, regulatory and catalytic protein modules that are required for a given biotransformation. In the future, successful development of these systems will allow the production of cell-free complex processes and will allow more sophisticated opportunities for enzyme-based biocatalysis such as versatile fuel cells, recycling of complex waste materials, multianalyte analytical biosensors and cell-free biosynthesis of biopolymers such as proteins and carbohydrates. A development that will support this approach to biocatalysis is nanobiotechnology and the availability of highly defined nanoparticles (.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
المجمع العلمي ينظّم ندوة حوارية حول مفهوم العولمة الرقمية في بابل
|
|
|