


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 16-5-2017
Date: 29-9-2020
Date: 2-11-2019
|
If we were walking on the beach, the plants shown below would look very different. They would be short and sticking out of the sand. When we see them this way, we do not immediately recognize them as beach plants. Often we need to look at the world around us in different ways to understand things better.

Figure 1 : Looking at things from a different perspective can often lead to deeper understanding. (CC BY-NC; CK-12)
The bonding scheme described by valence bond theory must account for molecular geometries as predicted by VSEPR theory. To do that, we must introduce a concept called hybrid orbitals.
Unfortunately, overlap of existing atomic orbitals (s, p, etc.) is not sufficient to explain some of the bonding and molecular geometries that are observed. Consider the element carbon and the methane (CH4) molecule. A carbon atom has the electron configuration of 1s22s22p2, meaning that it has two unpaired electrons in its 2p orbitals, as shown in the figure below.
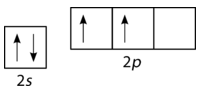
Figure 2 : Orbital configuration for carbon atom.
According to the description of valence bond theory so far, carbon would be expected to form only two bonds, corresponding to its two unpaired electrons. However, methane is a common and stable molecule, with four equivalent C−H
bonds. To account for this, one of the 2s electrons is promoted to the empty 2p
orbital (see figure below).
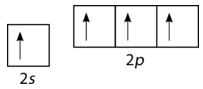
Figure 2 : Promotion of carbon s electron to empty p orbital.
Now, four bonds are possible. The promotion of the electron "costs" a small amount of energy, but recall that the process of bond formation is accompanied by a decrease in energy. The two extra bonds that can now be formed results in a lower overall energy and thus greater stability to the CH4 molecule. Carbon normally forms four bonds in most of its compounds.
The number of bonds is now correct, but the geometry is wrong. The three p orbitals (px, py, and pz) are oriented at 90o relative to one another. However, as was seen from VSEPR theory, the observed H−C−H bond angle in the tetrahedral CH4 molecule is actually 109.5o. Therefore, the methane molecule cannot be adequately represented by simple overlap of the 2s and 2p orbitals of carbon with the 1s orbitals of each hydrogen atom.
To explain the bonding in methane, it is necessary to introduce the concept of hybridization and hybrid atomic orbitals. Hybridization is the mixing of the atomic orbitals in an atom to produce a set of hybrid orbitals. When hybridization occurs, it must do so as a result of the mixing of nonequivalent orbitals. In other words, s
and p orbitals can hybridize but p orbitals cannot hybridize with other p orbitals. Hybrid orbitals are the atomic orbitals obtained when two or more nonequivalent orbitals from the same atom combine in preparation for bond formation. In the current case of carbon, the single 2s orbital hybridizes with the three 2p orbitals to form a set of four hybrid orbitals, called sp3 hybrids (see figure below).
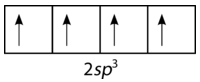
Figure 3 : Carbon sp3 hybrid orbitals.
The sp3 hybrids are all equivalent to one another. Spatially, the hybrid orbitals point towards the four corners of a tetrahedron (see figure below).

Figure 4 : The process of sp3 hybridization is the mixing of an s orbital with a set of three p orbitals to form a set of four sp3 hybrid orbitals. Each large lobe of the hybrid orbitals points to one corner of a tetrahedron. The four lobes of each of the sp3 hybrid orbitals then overlap with the normal unhybridized 1s orbitals of each hydrogen atoms to form the tetrahedral methane molecule.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|