


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية | Naming Binary Ionic Compounds with a Metal That Forms More Than One Type of Cation |
|
|
|
Read More
Date: 27-7-2020
Date: 20-11-2020
Date: 26-5-2019
|
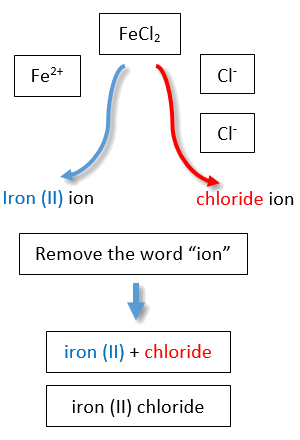
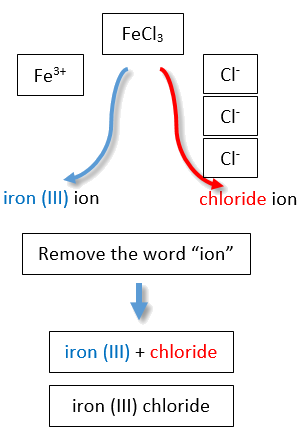
If you are given a formula for an ionic compound whose cation can have more than one possible charge, you must first determine the charge on the cation before identifying its correct name. For example, consider FeCl2 and FeCl3 . In the first compound, the iron ion has a 2+ charge because there are two Cl− ions in the formula (1− charge on each chloride ion). In the second compound, the iron ion has a 3+ charge, as indicated by the three Cl− ions in the formula. These are two different compounds that need two different names. By the Stock system, the names are iron(II) chloride and iron(III) chloride (Figure 1 ).
Table 1: Naming the FeCl2 and FeCl3 Compounds in the Modern/Stock System.
| Name of cation (metal) + (Roman Numeral in parenthesis) + Base name of anion (nonmetal) and -ide | |
 |
 |
If we were to use the stems and suffixes of the common system, the names would be ferrous chloride and ferric chloride, respectively (Figure 5.7.3
) .
Table 2 : Naming the FeCl2 and FeCl3 Compounds in the Old/Common System
| "Old" base name of cation (metal) and -ic or -ous + Base name of anion (nonmetal) and -ide | |
|
-ous (for ions with lower charge)
|
-ic (for ions with higher charge)
|



|
|
|
|
"إنقاص الوزن".. مشروب تقليدي قد يتفوق على حقن "أوزيمبيك"
|
|
|
|
|
|
|
الصين تحقق اختراقا بطائرة مسيرة مزودة بالذكاء الاصطناعي
|
|
|
|
|
|
|
قسم شؤون المعارف ووفد من جامعة البصرة يبحثان سبل تعزيز التعاون المشترك
|
|
|