


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 13-12-2019
Date: 25-7-2018
Date: 16-12-2019
|
Secondary structure of protien
Secondary structure is three-dimensional, but is a local phenomenon, confined to a relatively short stretch of amino acids. For the most part, there are three important elements of secondary structure: helices, beta-sheets, and loops. In a helix, the main chain of the protein adopts the shape of a clockwise spiral staircase, and the side chains point out laterally.
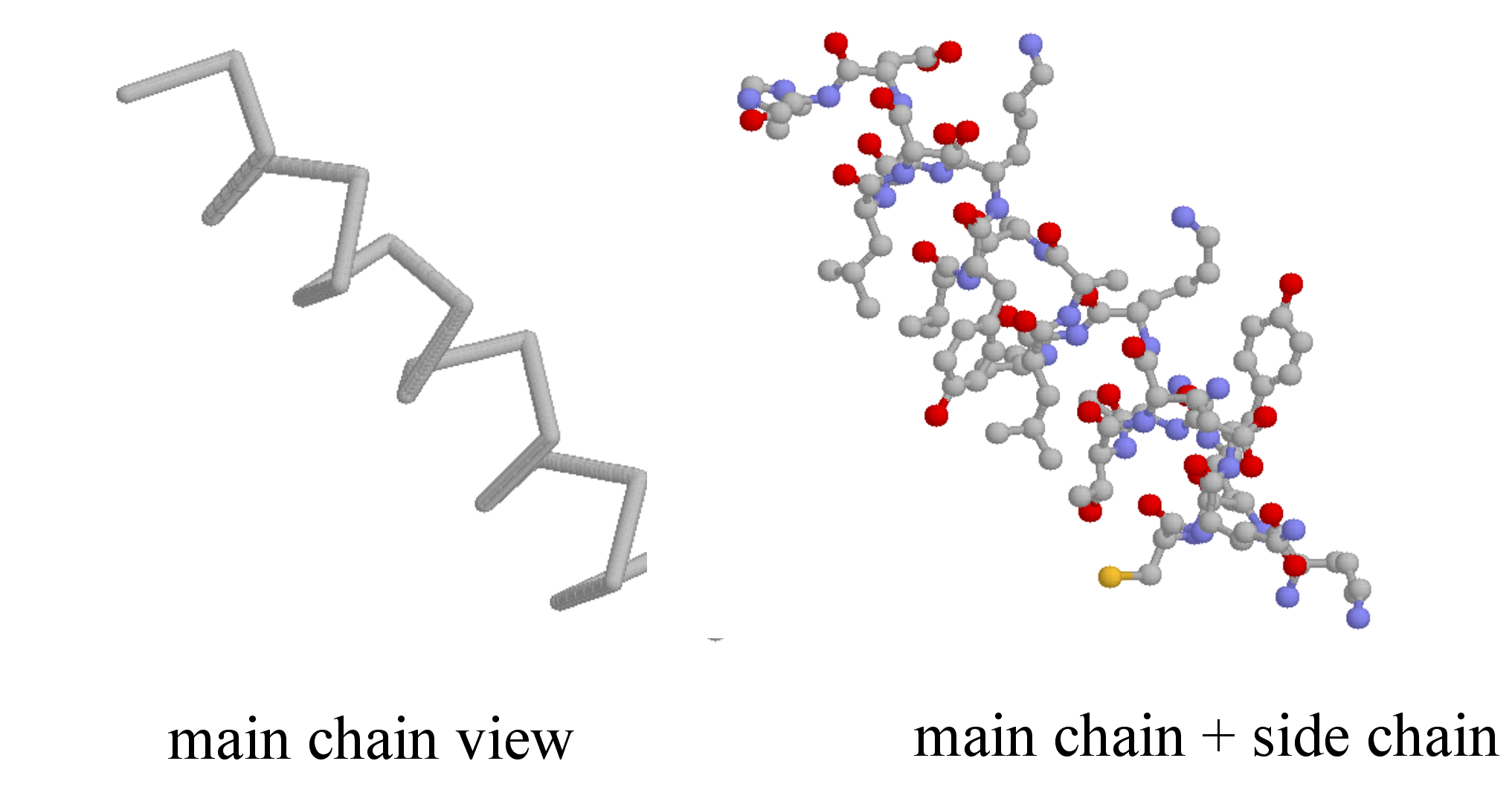
In a beta-sheet (or beta-strand) structure, two sections of protein chain are aligned side-by-side in an extended conformation. The figure below shows two different views of the same beta-sheet: in the left-side view, the two regions of protein chain are differentiated by color.

Loops are relatively disordered segments of protein chain, but often assume a very ordered structure when in contact with a second protein or a smaller organic compound.
Both helix and the beta-sheet structures are held together by very specific hydrogen-bonding interactions between the amide nitrogen on one amino acid and the carbonyl oxygen on another. The hydrogen bonding pattern in a section of a beta-strand is shown below.
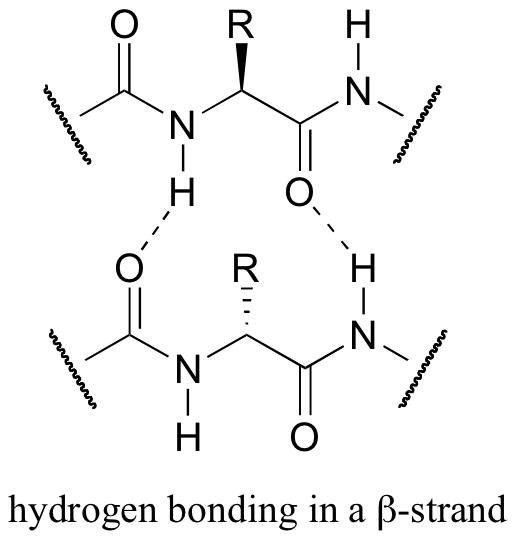
Secondary structure refers to the shape of a folding protein due exclusively to hydrogen bonding between its backbone amide and carbonyl groups. Secondary structure does not include bonding between the R-groups of amino acids, hydrophobic interactions, or other interactions associated with tertiary structure. The two most commonly encountered secondary structures of a polypeptide chain are α-helices and beta-pleated sheets. These structures are the first major steps in the folding of a polypeptide chain, and they establish important topological motifs that dictate subsequent tertiary structure and the ultimate function of the protein.



|
|
|
|
"عادة ليلية" قد تكون المفتاح للوقاية من الخرف
|
|
|
|
|
|
|
ممتص الصدمات: طريقة عمله وأهميته وأبرز علامات تلفه
|
|
|
|
|
|
|
المجمع العلمي للقرآن الكريم يقيم جلسة حوارية لطلبة جامعة الكوفة
|
|
|