


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 4-9-2019
Date: 26-2-2016
Date: 20-10-2020
|
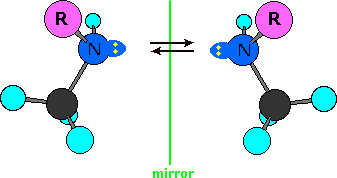
Single-bonded nitrogen is pyramidal in shape, with the non-bonding electron pair pointing to the unoccupied corner of a tetrahedral region. Since the nitrogen in these compounds is bonded to three different groups, its configuration is chiral. The non-identical mirror-image configurations are illustrated in the following diagram (the remainder of the molecule is represented by R, and the electron pair is colored yellow). If these configurations were stable, there would be four additional stereoisomers of ephedrine and pseudoephedrine. However, pyramidal nitrogen is normally not configurationally stable. It rapidly inverts its configuration (equilibrium arrows) by passing through a planar, sp2-hybridized transition state, leading to a mixture of interconverting R and S configurations. If the nitrogen atom were the only chiral center in the molecule, a 50:50 (racemic) mixture of R and S configurations would exist at equilibrium. If other chiral centers are present, as in the ephedrin isomers, a mixture of diastereomers will result. The take-home message is that nitrogen does not contribute to isolable stereoisomers.
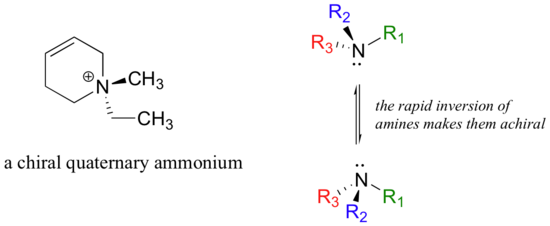
Asymmetric quaternary ammonium groups are also chiral. Amines, however, are not chiral, because they rapidly invert, or turn ‘inside out’, at room temperature.
The phosphorus center of phosphate ion and organic phosphate esters, for example, is tetrahedral, and thus is potentially a stereocenter.

We will see in chapter 10 how researchers, in order to investigate the stereochemistry of reactions at the phosphate center, incorporated sulfur and/or 17O and 18O isotopes of oxygen (the ‘normal’ isotope is 16O) to create chiral phosphate groups. Phosphate triesters are chiral if the three substituent groups are different.



|
|
|
|
"إنقاص الوزن".. مشروب تقليدي قد يتفوق على حقن "أوزيمبيك"
|
|
|
|
|
|
|
الصين تحقق اختراقا بطائرة مسيرة مزودة بالذكاء الاصطناعي
|
|
|
|
|
|
|
قسم شؤون المعارف ووفد من جامعة البصرة يبحثان سبل تعزيز التعاون المشترك
|
|
|