
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 9-7-2020
Date: 23-3-2017
Date: 12-2-2018
|
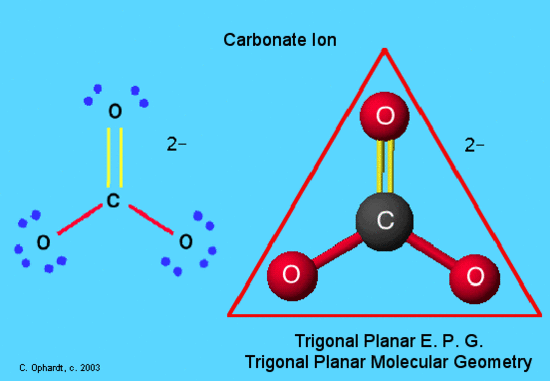
In this example, CO32-, the Lewis diagram shows carbon at the center with no lone electron pairs.
The Lewis diagram is as follows:
C = 4 e-
O = 6e- x 3 = 18e-
2- charge = 2e-
Total electrons = 24
One double bond is needed to give carbon an octet. The carbon and oxygen are bonded through a double bond which counts as "one electron pair" and two single bonded oxygens. Hence the molecule has three electron pairs and is trigonal planar geometry. Carbonate ion is present in limestone as calcium carbonate. A form of it, bicarbonate, is in baking soda and baking powder.



|
|
|
|
4 أسباب تجعلك تضيف الزنجبيل إلى طعامك.. تعرف عليها
|
|
|
|
|
|
|
أكبر محطة للطاقة الكهرومائية في بريطانيا تستعد للانطلاق
|
|
|
|
|
|
|
أصواتٌ قرآنية واعدة .. أكثر من 80 برعماً يشارك في المحفل القرآني الرمضاني بالصحن الحيدري الشريف
|
|
|