


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 2-2-2018
Date: 8-3-2019
Date: 27-4-2019
|
Physical properties
Table 1.1 lists selected physical properties of the group 14 elements. A comparison with Table 12.1 shows there to be some similarities in trends down groups 13 and 14. Ionization energies and cation formation On descending group 14, the trends in ionization energies reveal two particular points:
The sums of the first four ionization energies for any element suggest that it is unlikely that M4+ ions are formed. For example, although both SnF4 and PbF4 are non-volatile solids, neither has a symmetrical lattice structure in the solid state. Both SnO2 and PbO2 adopt the rutile lattice, but the fact that PbO2 is brown argues against a formulation of Pb4+(O2-)2. Agreement between values of lattice energies determined using a Born–Haber cycle and calculated from an electrostatic model is good for SnO2, but is poor for PbO2. Thus, values of the M4+ ionic radii (Table 1.1) should be treated with some caution.
Table 1.1 Some physical properties of the group 14 elements, M, and their ions.
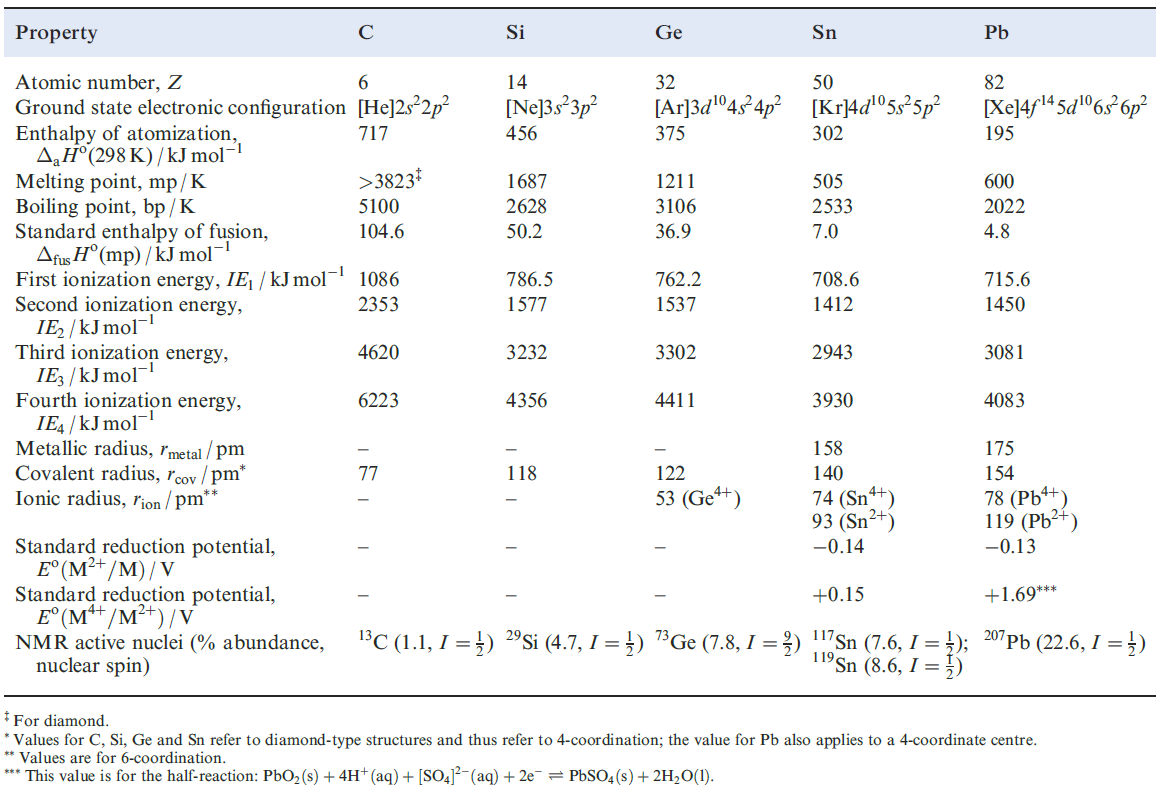
Aqueous solution chemistry involving cations of the group 14 elements is restricted mainly to Sn and Pb , and so Table 1.1 gives Eo values only for these metals.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
المجمع العلمي ينظّم ندوة حوارية حول مفهوم العولمة الرقمية في بابل
|
|
|