
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 23-2-2018
Date: 8-3-2019
Date: 30-4-2019
|
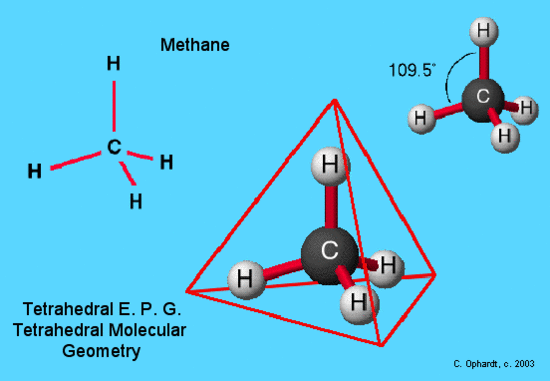
An example of tetrahedral electron pair geometry and molecular geometry is CH4. The carbon has 4 valence electrons and thus needs 4 more electrons from four hydrogen atoms to complete its octet. The hydrogen atoms are as far apart as possible at 109o bond angle. This is tetrahedral geometry. The molecule is three dimensional. Methane is the simplest hydrocarbon molecule present in natural gas. This molecule provides the basis for the tetrahedral geometries at each carbon in a hydrocarbon chain.



|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
العتبة العباسية المقدسة تستعد لإطلاق الحفل المركزي لتخرج طلبة الجامعات العراقية
|
|
|