


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 28-1-2018
Date: 28-1-2018
Date: 10-12-2018
|
Imagine that this ion is placed next to a positive ion. The positive ion attracts the delocalized electrons in the carbonate ion towards itself. The carbonate ion becomes polarized. The diagram shows what happens with an ion from Group 2, carrying two positive charges:
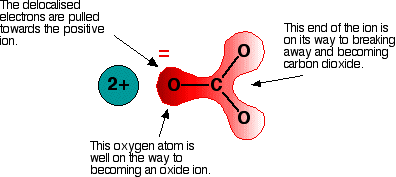
If this system is heated, the carbon dioxide breaks free, leaving a metal oxide. The amount of heat required depends on how polarized the ion was. If it is highly polarized, less heat is required than if it is only slightly polarized. If the positive ion only has one positive charge, the polarizing effect is lessened. This is why the Group 1 compounds are more thermally stable than those in Group 2. The Group 1 compound must be heated more because the carbonate ion is less polarized by a singly-charged positive ion.
The smaller the positive ion, the higher the charge density, and the greater the effect on the carbonate ion. As the positive ions get bigger down the group, they have less effect on the carbonate ions near them. To compensate, the compound must be heated more in order to force the carbon dioxide to break off and leave the metal oxide.



|
|
|
|
للعاملين في الليل.. حيلة صحية تجنبكم خطر هذا النوع من العمل
|
|
|
|
|
|
|
"ناسا" تحتفي برائد الفضاء السوفياتي يوري غاغارين
|
|
|
|
|
|
|
ملاكات العتبة العباسية المقدسة تُنهي أعمال غسل حرم مرقد أبي الفضل العباس (عليه السلام) وفرشه
|
|
|