


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 21-1-2022
Date: 10-7-2018
Date: 27-8-2018
|
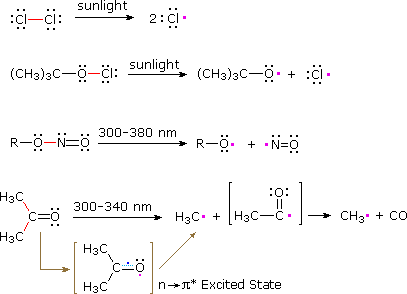
Compounds having absorption bands in the visible or near ultraviolet spectrum may be electronically excited to such a degree that weak covalent bonds undergo homolysis. Examples include the halogens Cl2, Br2 & I2 (bond dissociation energies are 58, 46 & 36 kcal/mole respectively), alkyl hypochlorites, nitrite esters and ketones. Equations illustrating these radical producing reactions are displayed below. The covalent bonds that undergo homolysis are colored red, and the unpaired electrons in the resulting radicals are colored pink. Ketones undergo n to π* electronic excitation near 300 nm. The resulting excited state is a diradical in which one of the odd electrons is localized on the oxygen atom. Cleavage of an alkyl group may then take place.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
المجمع العلمي ينظّم ندوة حوارية حول مفهوم العولمة الرقمية في بابل
|
|
|