
Polar Reactions
 المؤلف:
John McMurry
المؤلف:
John McMurry
 المصدر:
Organic Chemistry
المصدر:
Organic Chemistry
 الجزء والصفحة:
9th. p 155
الجزء والصفحة:
9th. p 155
 13-5-2017
13-5-2017
 7966
7966
Polar Reactions
Polar reactions occur because of the electrical attraction between positively polarized and negatively polarized centers on functional groups in molecules. Most organic compounds are electrically neutral; they have no net charge, either positive or negative. however, that certain bonds within a molecule, particularly the bonds in functional groups, are polar.
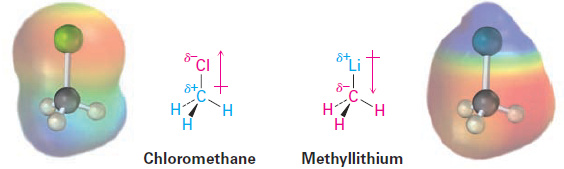
Bond polarity is a consequence of an unsymmetrical electron distribution in a bond and is due to the difference in electronegativity of the bonded atoms. Elements such as oxygen, nitrogen, fluorine, and chlorine are more electronegative than carbon, so a carbon atom bonded to one of these atoms has a partial positive charge (δ+). Conversely, metals are less electronegative than carbon, so a carbon atom bonded to a metal has a partial negative charge (δ-). Electrostatic potential maps of chloromethane and methyllithium illustrate these charge distributions, showing that the carbon atom in chloromethane is electron-poor (blue) while the carbon in methyllithium is electron-rich (red).
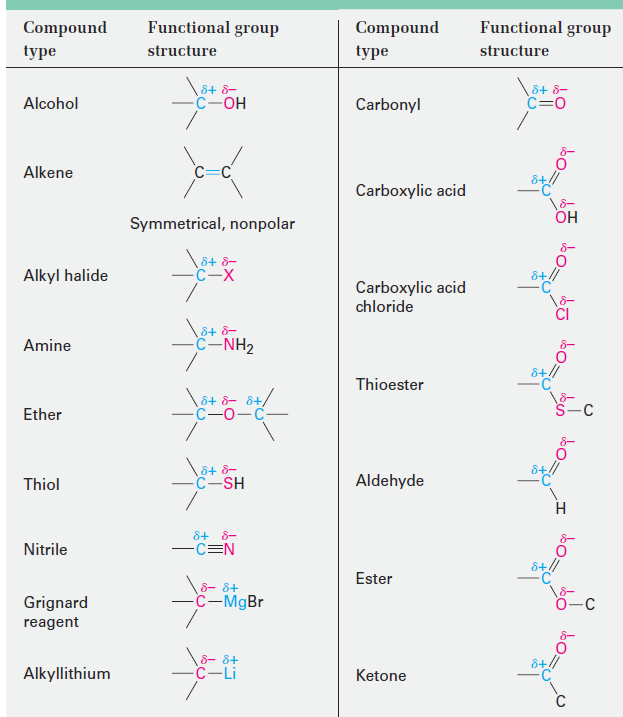
The polarity patterns of some common functional groups are shown in Table 1.1. Note that carbon is always positively polarized except when bonded to a metal.
This discussion of bond polarity is oversimplified in that we’ve considered only bonds that are inherently polar due to differences in electronegativity. Polar bonds can also result from the interaction of functional groups with acids or bases. Take an alcohol such as methanol, for example. In neutral methanol, the carbon atom is somewhat electron-poor because the electronegative oxygen attracts the electrons in the C-O bond. On protonation of the methanol oxygen by an acid, however, a full positive charge on oxygen attracts the electrons in the C-O bond much more strongly and makes the carbon much more electron-poor. We’ll see numerous examples throughout this book of reactions that are catalyzed by acids because of the resultant increase in bond polarity upon protonation.

Table 1.1 Polarity Patterns in Some Common Functional Groups
Yet a further consideration is the polarizability (as opposed to polarity) of atoms in a molecule. As the electric field around a given atom changes because of changing interactions with solvent or other polar molecules nearby, the electron distribution around that atom also changes. The measure of this response to an external electrical influence is called the polarizability of the atom. Larger atoms with more loosely held electrons are more polarizable, and smaller atoms with fewer, tightly held electrons are less polarizable. Thus, sulfur is more polarizable than oxygen, and iodine is more polarizable than chlorine. The effect of this higher polarizability of sulfur and iodine is that carbon–sulfur and carbon–iodine bonds, although nonpolar according to electronegativity values nevertheless usually react as if they were polar.

What does functional-group polarity mean with respect to chemical reactivity? Because unlike charges attract, the fundamental characteristic of all polar organic reactions is that electron-rich sites react with electron-poor sites. Bonds are made when an electron-rich atom donates a pair of electrons to an electron-poor atom, and bonds are broken when one atom leaves with both electrons from the former bond.
A curved arrow shows where electrons move when reactant bonds are broken and product bonds are formed. This means that an electron pair moves from the atom (or bond) at the tail of the arrow to the atom at the head of the arrow during the reaction.
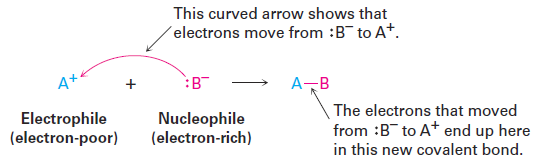
In referring to the electron-rich and electron-poor species involved in polar reactions, chemists use the words nucleophile and electrophile. A nucleophile is a substance that is “nucleus-loving.” (Remember that a nucleus is positively charged.) A nucleophile has a negatively polarized, electron-rich atom and can form a bond by donating a pair of electrons to a positively polarized, electronpoor atom. Nucleophiles can be either neutral or negatively charged; ammonia, water, hydroxide ion, and chloride ion are examples. An electrophile, by contrast, is “electron-loving.” An electrophile has a positively polarized, electronpoor atom and can form a bond by accepting a pair of electrons from a nucleophile. Electrophiles can be either neutral or positively charged. Acids (H1 donors), alkyl halides, and carbonyl compounds are examples (Figure 1.1).
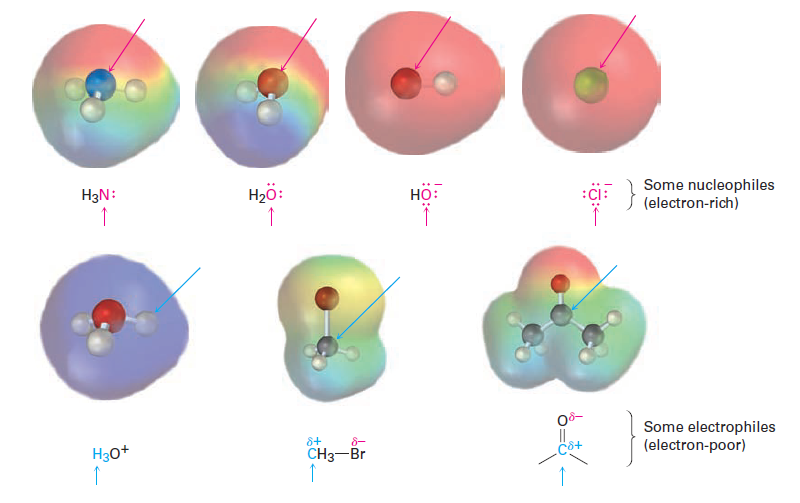
Figure 1.1 Some nucleophiles and electrophiles. Electrostatic potential maps identify the nucleophilic (negative) and electrophilic (positive) atoms.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


