
Peptides Are Chains of Amino Acids
 المؤلف:
David L. Nelson, Michael M. Cox
المؤلف:
David L. Nelson, Michael M. Cox
 المصدر:
Book or Source : Lehninger Principles of Biochemistry 6th ed 2012
المصدر:
Book or Source : Lehninger Principles of Biochemistry 6th ed 2012
 الجزء والصفحة:
p 85
الجزء والصفحة:
p 85
 9-4-2017
9-4-2017
 3202
3202
Peptides Are Chains of Amino Acids
Two amino acid molecules can be covalently joined through a substituted amide linkage, termed a peptide bond, to yield a dipeptide. Such a linkage is formed by removal of the elements of water (dehydration) from the α-carboxyl group of one amino acid and the α-amino group of another (Fig. 1.1). Peptide bond formation is an example of a condensation reaction, a common class of reactions in living cells. Under standard biochemical conditions, the equilibrium for the reaction shown in Figure 1.1 favors the amino acids over the dipeptide. To make the reaction thermodynamically more favorable, the carboxyl group must be chemically modified or activated so that the hydroxyl group can be more readily eliminated. A chemical approach to this problem is outlined later in this chapter. Three amino acids can be joined by two peptide bonds to form a tripeptide; similarly, amino acids can be linked to form tetrapeptides, pentapeptides, and so forth. When a few amino acids are joined in this fashion, the structure is called an oligopeptide. When many amino acids are joined, the product is called a polypeptide. Proteins may have thousands of amino acid residues. Although the terms “protein” and “polypeptide” are sometimes used interchangeably, molecules referred to as polypeptides generally have molecular weights below 10,000, and those called proteins have higher molecular weights. Figure 1.2 shows the structure of a pentapeptide. As already noted, an amino acid unit in a peptide is often called a residue (the part left over after losing a hydrogen atom from its amino group and the hydroxyl moiety from its carboxyl group). In a peptide, the amino acid residue at the end with a free α-amino group is the amino-terminal (or N-terminal) residue; the residue
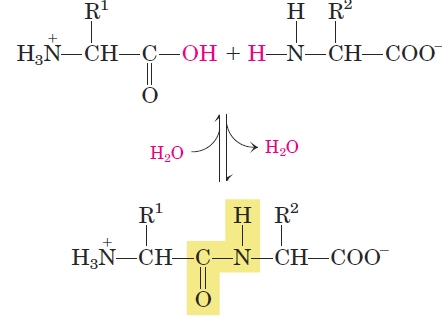
FIGURE 1.1 Formation of a peptide bond by condensation. The α-amino group of one amino acid (with R1 --> group) acts as a nucleophile to displace the hydroxyl group of another amino acid (with R2--> group), forming a peptide bond (shaded in yellow). Amino groups are good nucleophiles, but the hydroxyl group is a poor leaving group and is not readily displaced. At physiological pH, the reaction shown does not occur to any appreciable extent.
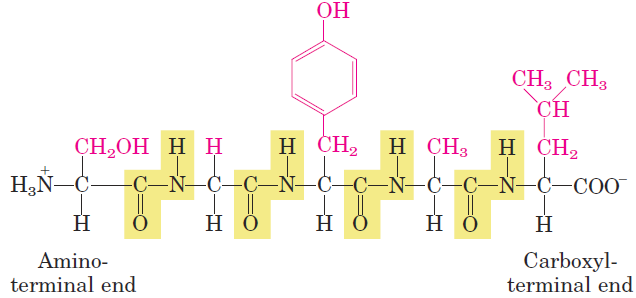
FIGURE 1.2 The pentapeptide serylglycyltyrosylalanylleucine, or Ser–Gly–Tyr–Ala–Leu. Peptides are named beginning with the aminoterminal residue, which by convention is placed at the left. The peptide bonds are shaded in yellow; the R groups are in red.
at the other end, which has a free carboxyl group, is the carboxyl-terminal (C-terminal) residue. Although hydrolysis of a peptide bond is an exergonic reaction, it occurs slowly because of its high activation energy. As a result, the peptide bonds in proteins are quite stable, with an average half-life (t1/2) of about 7 years under most intracellular conditions.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


