
Hydroformylation
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص698-700
الجزء والصفحة:
ص698-700
 2025-10-18
2025-10-18
 296
296
Hydroformylation
Key point: The mechanism of hydrocarbonylation is thought to involve a pre-equilibrium in which octa carbonyldicobalt combines with hydrogen at high pressure to give a monometallic species that brings about the actual hydrocarbonylation reaction. In a hydroformylation reaction, an alkene, CO, and H2 react to form an aldehyde containing one more C atom than in the original alkene:
RCH=CH2+CO+H2 → RCH2CH2CHO
The term ‘hydroformylation’ derived from the idea that the product resulted from the addition of methanal (formaldehyde, HCHO) to the alkene, and the name has stuck even though experimental data indicate a different mechanism. A less common but more ap propriate name is hydrocarbonylation. Both cobalt and rhodium complexes are used as catalysts. Aldehydes produced by hydroformylation are normally reduced to alcohols that are used as solvents and plasticizers, and in the synthesis of detergents. The scale of pro duction is enormous, amounting to millions of tonnes annually. The general mechanism of cobalt-carbonyl-catalysed hydroformylation was proposed in 1961 by Heck and Breslow by analogy with reactions familiar from organometallic chemistry (Fig. 26.7). Their general mechanism is still invoked, but has proved difficult to verify in detail. In the proposed mechanism, a pre-equilibrium is established in which octacarbonyldicobalt combines with hydrogen at high pressure to yield the known tetracarbonylhydridocobalt complex (A):
[Co2(CO)8] +H2 → 2[Co (CO)4H]
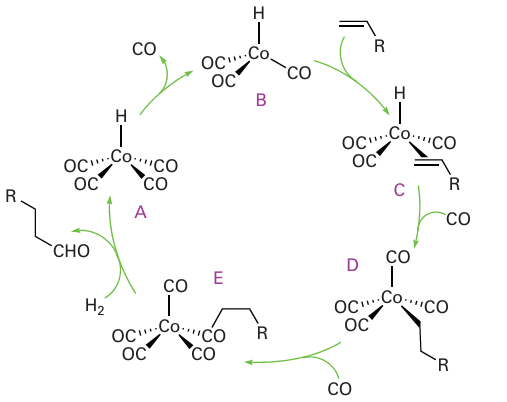
Figure 26.7 The catalytic cycle for the hydroformylation of alkenes by a cobalt carbonyl catalyst.
This complex, it is proposed, loses CO to produce the coordinatively unsaturated complex [Co (CO)3 H] (B):
[Co(CO)4H]→ [Co(CO)3H]+CO
It is thought that [Co (CO)3H] then coordinates an alkene, producing (C) in Fig. 26.7, whereupon the coordinated hydrido ligand migrates onto the alkene, and CO recoordi nates. The product at this stage is a normal alkyl complex (D). In the presence of CO at high pressure, (D) undergoes migratory insertion and coordinates another CO, yielding the acyl complex (E), which has been observed by IR spectroscopy under catalytic reaction conditions. The formation of the aldehyde product is thought to occur by attack of either H2 (as depicted in Fig. 26.7) or the strongly acidic complex [Co (CO)4H] to yield an aldehyde and generate [Co (CO)4H] or [Co2(CO)8], respectively. Either of these complexes will regenerate the coordinatively unsaturated [Co (CO)3H]. A significant portion of branched aldehyde is also formed in the cobalt-catalysed hydroformylation. This product may result from a 2-alkylcobalt intermediate formed when reaction of (C) leads to an isomer of (D), with hydrogenation then yielding a branched aldehyde as set out in Fig. 26.8. When the linear aldehyde is required, such as for the syn thesis of biodegradable detergents, the isomerization can be suppressed by the addition of an alkylphosphine to the reaction mixture. One plausible explanation is that the replace ment of CO by a bulky ligand disfavours the formation of complexes of sterically crowded 2-alkenes:
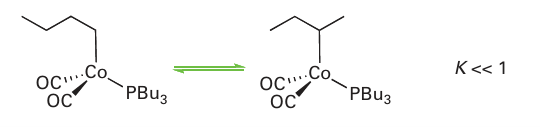
Here again we see an example of the powerful influence of ancillary ligands on catalysis. Another effective hydroformylation catalyst precursor is [Rh (CO)H(PPh3)3] (7), which loses a phosphine ligand to form the coordinatively unsaturated 16-electron complex [Rh (CO)H (PPh3 )2], which promotes hydroformylation at moderate temperatures and 1 atm. This behaviour contrasts with the cobalt carbonyl catalyst, which typically requires 150ºC and 250 atm. The rhodium catalyst is useful in the laboratory as it is effective under convenient conditions. Because it favours linear aldehyde products, it competes with the phosphine-modified cobalt catalyst in industry.
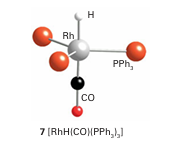
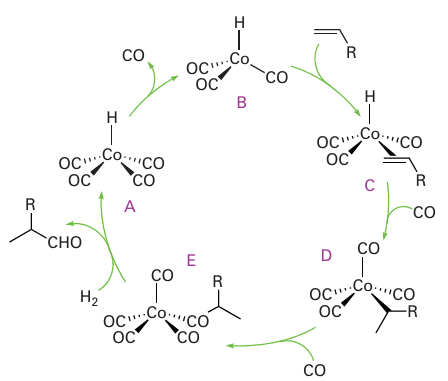
Figure 26.8 The formation of branched aldehydes in hydroformylation reactions occurs when the alkyl group is not terminally bound.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


