
Sulfur
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص404-405
الجزء والصفحة:
ص404-405
 2025-09-10
2025-09-10
 476
476
Sulfur
Key points: Sulfur is extracted as the element from underground deposits. It has many allotropic and polymorphic forms, including a metastable polymer, but its most stable form is the cyclic S8 molecule. Sulfur can be extracted from deposits of the element by the Frasch process, in which underground deposits are forced to the surface using superheated water and steam, and compressed air. The extracted S is molten and is allowed to cool in large basins. The process is energy intensive and commercial success depends on access to cheap water and energy. Extraction from natural gas and crude oil by the Claus process has become increasingly important. In this process, H2S is first oxidized in air at 1000-1400C. This step produces some SO2 that then reacts with the remaining H2S at 200-350C over a catalyst:
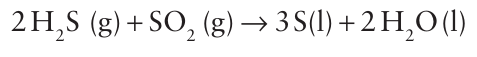
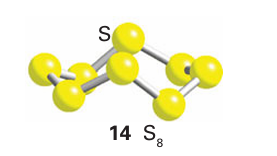
Unlike O, S (and all the heavier members of the group) tends to form single bonds with itself rather than double bonds because of the poor π overlap of its orbitals, which are held apart by the bulky atomic cores of neighbouring atoms. As a result, it aggregates into larger molecules or extended structures and hence is a solid at room temperature. Sulfur vapour, which is formed at high temperatures, consists partially of paramagnetic disulfur molecules, S2, that resemble O2 in having a triplet ground state and a formal double bond. The common yellow orthorhombic polymorph, α-S8, consists of crown-like eight-membered rings (14) and all other forms of S eventually revert to this form. Orthorhombic -sulfur is an electrical and thermal insulator. When it is heated to 93C, the packing of the S8 rings is modified and monoclinic ß-S8 forms. When molten sulfur that has been heated above 150C is cooled slowly, monoclinic -sulfur is formed. This polymorph consists of S8 rings like the and forms but the packing of the rings is more efficient, resulting in a higher density.
A note on good practice The various molecular entities that S forms are allotropes of the element. The various crystalline forms that these entities exist as are polymorphs.
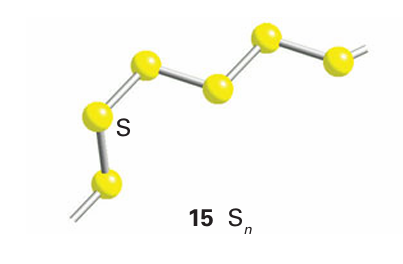
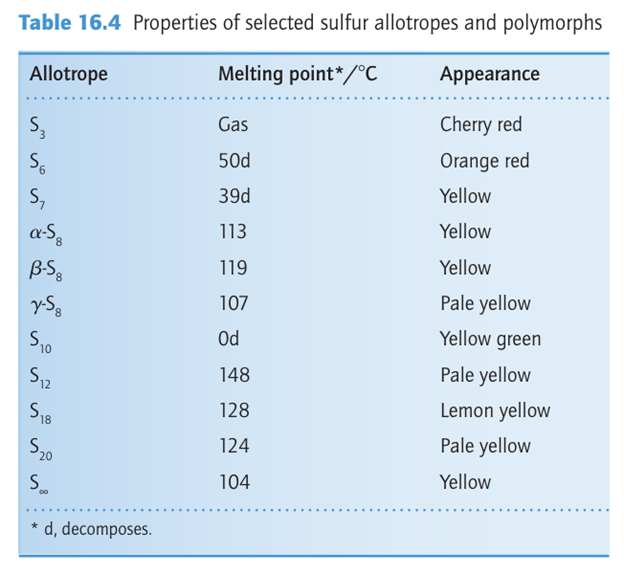
It is possible to synthesize and crystallize sulfur rings with from six to 20 S atoms (Table 16.4). An additional complexity is that some of these allotropes exist in several crystalline forms. For example, S7 is known in four crystalline forms and S18 is known in two. Orthorhombic sulfur melts at 113C; the yellow liquid darkens above 160C and becomes more viscous as the sulfur rings break open and polymerize. The resulting helical Sn poly mers (15) can be drawn from the melt and quenched to form metastable rubber-like materials that slowly revert to α-S8 at room temperature. In the gas phase, S2 and S3 are observed. S3 is a cherry red, angular molecule like ozone. The more stable speciesis the violet S2 molecule that, like O2, is doubly bonded with a bond dissociation energy of 421 kJ mol-1. Sulfur reacts directly with many elements at room or elevated temperatures. It ignites in F2 to form SF6 , reacts rapidly with Cl2 to form S2Cl2, and dissolves in Br2 to give S2Br2, which readily dissociates. It does not react with liquid I2, which can therefore be used as a low-temperature solvent for sulfur. Atomic sulfur, S, is extremely reactive, and triplet and singlet states are possible with different reactivities, as with O.
Most of the sulfur produced is used to manufacture sulfuric acid, H2SO4, which is one of the most important manufactured chemicals. Sulfuric acid has many uses, including the synthesis of fertilizers and in dilute aqueous solution as the electrolyte in lead acid batteries (Box 14.6). Sulfur is a component of gun powder (a mixture of potassium nitrate, KNO3, carbon, and sulfur). It is also used in the vulcanization of natural rubber.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


