
Reactivity of oxygen
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
405
الجزء والصفحة:
405
 2025-09-10
2025-09-10
 401
401
Reactivity of oxygen
Key point: The reactions of dioxygen are often thermodynamically favourable but sluggish. Oxygen is by no means an inert molecule, yet many of its reactions are sluggish (a point first made in connection with overpotentials in Section 5.18). For example, a solution of Fe+2 is only slowly oxidized by air even though the reaction is thermodynamically favourable. Several factors contribute to the appreciable activation energy of many reactions of O2. One factor is that, with weak reducing agents, single-electron transfer to O2 is mildly unfavourable thermodynamically:
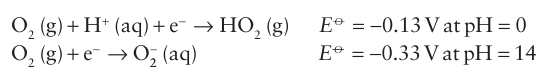
A single-electron reducing reagent must exceed these potentials for the reaction to be thermodynamically viable and must exceed them to achieve a significant rate. Second, the ground state of O2, with both π* orbitals singly occupied, is neither an effective Lewis acid nor an effective Lewis base, and therefore has little tendency to undergo displacement reactions with p-block Lewis bases or acids. Finally, the high bond energy of O2 (497 kJ mol-1) results in a high activation energy for reactions that de pend on its dissociation. Radical chain mechanisms can provide reaction paths that circumvent some of these activation barriers in combustion processes at elevated temperatures, and radical oxidations also occur in solution. In metalloenzymes (Section 27.10) O2 coordinates to metals such as Fe and Cu, and the en zyme catalyses the four-electron reduction of O2 to water.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


