

النبات

مواضيع عامة في علم النبات

الجذور - السيقان - الأوراق

النباتات الوعائية واللاوعائية

البذور (مغطاة البذور - عاريات البذور)

الطحالب

النباتات الطبية


الحيوان

مواضيع عامة في علم الحيوان

علم التشريح

التنوع الإحيائي

البايلوجيا الخلوية


الأحياء المجهرية

البكتيريا

الفطريات

الطفيليات

الفايروسات


علم الأمراض

الاورام

الامراض الوراثية

الامراض المناعية

الامراض المدارية

اضطرابات الدورة الدموية

مواضيع عامة في علم الامراض

الحشرات


التقانة الإحيائية

مواضيع عامة في التقانة الإحيائية


التقنية الحيوية المكروبية

التقنية الحيوية والميكروبات

الفعاليات الحيوية

وراثة الاحياء المجهرية

تصنيف الاحياء المجهرية

الاحياء المجهرية في الطبيعة

أيض الاجهاد

التقنية الحيوية والبيئة

التقنية الحيوية والطب

التقنية الحيوية والزراعة

التقنية الحيوية والصناعة

التقنية الحيوية والطاقة

البحار والطحالب الصغيرة

عزل البروتين

هندسة الجينات


التقنية الحياتية النانوية

مفاهيم التقنية الحيوية النانوية

التراكيب النانوية والمجاهر المستخدمة في رؤيتها

تصنيع وتخليق المواد النانوية

تطبيقات التقنية النانوية والحيوية النانوية

الرقائق والمتحسسات الحيوية

المصفوفات المجهرية وحاسوب الدنا

اللقاحات

البيئة والتلوث


علم الأجنة

اعضاء التكاثر وتشكل الاعراس

الاخصاب

التشطر

العصيبة وتشكل الجسيدات

تشكل اللواحق الجنينية

تكون المعيدة وظهور الطبقات الجنينية

مقدمة لعلم الاجنة


الأحياء الجزيئي

مواضيع عامة في الاحياء الجزيئي


علم وظائف الأعضاء


الغدد

مواضيع عامة في الغدد

الغدد الصم و هرموناتها

الجسم تحت السريري

الغدة النخامية

الغدة الكظرية

الغدة التناسلية

الغدة الدرقية والجار الدرقية

الغدة البنكرياسية

الغدة الصنوبرية

مواضيع عامة في علم وظائف الاعضاء

الخلية الحيوانية

الجهاز العصبي

أعضاء الحس

الجهاز العضلي

السوائل الجسمية

الجهاز الدوري والليمف

الجهاز التنفسي

الجهاز الهضمي

الجهاز البولي


المضادات الميكروبية

مواضيع عامة في المضادات الميكروبية

مضادات البكتيريا

مضادات الفطريات

مضادات الطفيليات

مضادات الفايروسات

علم الخلية

الوراثة

الأحياء العامة

المناعة

التحليلات المرضية

الكيمياء الحيوية

مواضيع متنوعة أخرى

الانزيمات
Collagen illustrates the role of posttranslational processing in protein maturation
المؤلف:
Peter J. Kennelly, Kathleen M. Botham, Owen P. McGuinness, Victor W. Rodwell, P. Anthony Weil
المصدر:
Harpers Illustrated Biochemistry
الجزء والصفحة:
32nd edition.p43-44
2025-02-20
995
Protein Maturation Often Involves Making & Breaking of Covalent Bonds
The maturation of proteins into their final structural state often involves the cleavage or formation (or both) of covalent bonds, a process of posttranslational modification. Many polypeptides are initially synthesized as larger precursors called proproteins. The “extra” polypeptide segments in these proproteins often serve as leader sequences that target a polypeptide to a particular organelle or facilitate its pas sage through a membrane or to provide secondary structural scaffolds to guide folding of the polypeptide. Other segments ensure that any potentially harmful activity, such as that of the proteases trypsin and chymotrypsin, remains dormant until they reach their intended destination. However, once these transient requirements are fulfilled, the now superfluous pep tide regions are removed by selective proteolysis. Other covalent modifications may add new chemical functionalities to a protein. The maturation of collagen illustrates both of these processes.
Collagen Is a Fibrous Protein
The fibrous proteins collagen, keratin, and myosin account for more than 25% of the protein mass in the human body. These fibrous proteins represent a primary source of structural strength for cells (ie, the cytoskeleton) and tissues (eg, the extracellular matrix). Skin derives its strength and flexibility from an intertwined mesh of collagen and keratin fibers, while bones and teeth are buttressed by an underlying network of collagen fibers analogous to steel strands in reinforced concrete. Collagen also is present in connective tissues such as ligaments and tendons. The high degree of tensile strength required to fulfill these structural roles derives from collagen’s elongated form and high degree of crosslinking.
Collagen Forms a Unique Triple Helix
A mature collagen fiber forms an elongated rod with an axial ratio of about 200. Each fiber is comprised of three intertwined polypeptide strands, which twist to the left, wrapping around one another in a right-handed fashion to form the unique collagen triple helix (Figure 1). The opposing handedness of this superhelix and its component polypeptides makes the collagen triple helix highly resistant to unwinding—a principle also applied to the steel cables of suspension bridges. A collagen triple helix has 3.3 residues per turn and a rise per residue nearly twice that of an α helix. The R groups of each polypeptide strand of the triple helix pack so closely that, in order to fit, one of the three must be H. Thus, every third amino acid residue in colla gen is a glycine residue. Staggering of the three strands provides appropriate positioning of the requisite glycines throughout the helix. Collagen is also rich in proline and hydroxyproline, yielding a repetitive Gly-X-Y pattern (see Figure 1) in which Y generally is proline or hydroxyproline.
Collagen triple helices are stabilized by hydrogen bonds between residues in different polypeptide chains, a process helped by the hydroxyl groups of hydroxyprolyl residues. Additional stability is provided by covalent cross-links formed between modified lysyl residues both within and between polypeptide chains.
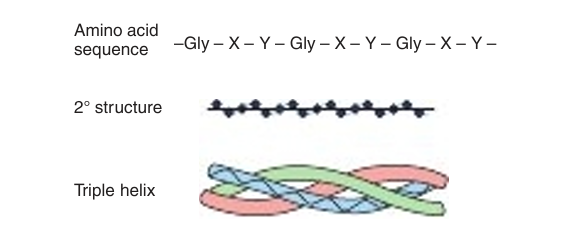
Fig1. Primary, secondary, and tertiary structures of collagen.
Collagen Is Synthesized as a Larger Precursor
Collagen is initially synthesized as a larger precursor poly peptide, procollagen. Numerous prolyl and lysyl residues of procollagen are hydroxylated by prolyl hydroxylase and lysyl hydroxylase, enzymes that require ascorbic acid . Hydroxyprolyl and hydroxylysyl residues provide additional hydrogen-bonding capability that stabilizes the mature protein. In addition, glucosyl and galactosyl transferases attach glucosyl or galactosyl residues to the hydroxyl groups of specific hydroxylysyl residues.
The central portion of the precursor polypeptide then associates with other molecules to form the characteristic triple helix. This process is accompanied by the removal of the globular amino terminal and carboxyl terminal extensions of the precursor polypeptide by selective proteolysis. Certain lysyl residues are modified by lysyl oxidase, a copper containing protein that converts ε-amino groups to aldehydes. The aldehydes can either undergo an aldol condensation to form a C=C double bond or form a Schiff base (eneimine) with the ε-amino group of an unmodified lysyl residue, which is subsequently reduced to form a C—N single bond. These covalent bonds cross-link the individual polypeptides and imbue the fiber with exceptional strength and rigidity.
Nutritional & Genetic Disorders Can Impair Collagen Maturation
The complex series of events in collagen maturation illustrate the consequences of incomplete polypeptide maturation. The best-known defect in collagen biosynthesis is scurvy, which results from a dietary deficiency of the vitamin C required by prolyl and lysyl hydroxylases. The consequent deficit in the number of hydroxyproline and hydroxylysine residues undermines the conformational stability of collagen fibers, leading to bleeding gums, swelling joints, poor wound healing, and ultimately death. Menkes syndrome, characterized by kinky hair and growth retardation, is brought on by a dietary deficiency of the copper required by lysyl oxidase, which catalyzes a key step in the formation of the covalent cross-links that strengthen collagen fibers.
Genetic disorders of collagen biosynthesis include several forms of osteogenesis imperfecta, characterized by fragile bones. In the Ehlers-Danlos syndrome, a group of connective tissue disorders that involve impaired integrity of supporting structures, defects in the genes that encode α collagen-1, procollagen N-peptidase, or lysyl hydroxylase result in mobile joints and skin abnormalities .
 الاكثر قراءة في الكيمياء الحيوية
الاكثر قراءة في الكيمياء الحيوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















