

النبات

مواضيع عامة في علم النبات

الجذور - السيقان - الأوراق

النباتات الوعائية واللاوعائية

البذور (مغطاة البذور - عاريات البذور)

الطحالب

النباتات الطبية


الحيوان

مواضيع عامة في علم الحيوان

علم التشريح

التنوع الإحيائي

البايلوجيا الخلوية


الأحياء المجهرية

البكتيريا

الفطريات

الطفيليات

الفايروسات


علم الأمراض

الاورام

الامراض الوراثية

الامراض المناعية

الامراض المدارية

اضطرابات الدورة الدموية

مواضيع عامة في علم الامراض

الحشرات


التقانة الإحيائية

مواضيع عامة في التقانة الإحيائية


التقنية الحيوية المكروبية

التقنية الحيوية والميكروبات

الفعاليات الحيوية

وراثة الاحياء المجهرية

تصنيف الاحياء المجهرية

الاحياء المجهرية في الطبيعة

أيض الاجهاد

التقنية الحيوية والبيئة

التقنية الحيوية والطب

التقنية الحيوية والزراعة

التقنية الحيوية والصناعة

التقنية الحيوية والطاقة

البحار والطحالب الصغيرة

عزل البروتين

هندسة الجينات


التقنية الحياتية النانوية

مفاهيم التقنية الحيوية النانوية

التراكيب النانوية والمجاهر المستخدمة في رؤيتها

تصنيع وتخليق المواد النانوية

تطبيقات التقنية النانوية والحيوية النانوية

الرقائق والمتحسسات الحيوية

المصفوفات المجهرية وحاسوب الدنا

اللقاحات

البيئة والتلوث


علم الأجنة

اعضاء التكاثر وتشكل الاعراس

الاخصاب

التشطر

العصيبة وتشكل الجسيدات

تشكل اللواحق الجنينية

تكون المعيدة وظهور الطبقات الجنينية

مقدمة لعلم الاجنة


الأحياء الجزيئي

مواضيع عامة في الاحياء الجزيئي


علم وظائف الأعضاء


الغدد

مواضيع عامة في الغدد

الغدد الصم و هرموناتها

الجسم تحت السريري

الغدة النخامية

الغدة الكظرية

الغدة التناسلية

الغدة الدرقية والجار الدرقية

الغدة البنكرياسية

الغدة الصنوبرية

مواضيع عامة في علم وظائف الاعضاء

الخلية الحيوانية

الجهاز العصبي

أعضاء الحس

الجهاز العضلي

السوائل الجسمية

الجهاز الدوري والليمف

الجهاز التنفسي

الجهاز الهضمي

الجهاز البولي


المضادات الميكروبية

مواضيع عامة في المضادات الميكروبية

مضادات البكتيريا

مضادات الفطريات

مضادات الطفيليات

مضادات الفايروسات

علم الخلية

الوراثة

الأحياء العامة

المناعة

التحليلات المرضية

الكيمياء الحيوية

مواضيع متنوعة أخرى

الانزيمات
Chromatin Remodeling Is an Active Process
المؤلف:
JOCELYN E. KREBS, ELLIOTT S. GOLDSTEIN and STEPHEN T. KILPATRICK
المصدر:
LEWIN’S GENES XII
الجزء والصفحة:
10-6-2021
4425
Chromatin Remodeling Is an Active Process
KEY CONCEPTS
- Numerous chromatin-remodeling complexes use energy provided by hydrolysis of ATP.
- All remodeling complexes contain a related ATPase catalytic subunit and are grouped into subfamilies containing more closely related ATPase subunits.
- Remodeling complexes can alter, slide, or displace nucleosomes.
- Some remodeling complexes can exchange one histone for another in a nucleosome.
Transcriptional activators face a challenge when trying to bind to their recognition sites in eukaryotic chromatin. FIGURE 1 illustrates two general states that can exist at a eukaryotic promoter. In the inactive state, nucleosomes are present, and they prevent basal factors and RNA polymerase from binding. In the active state, the basal apparatus occupies the promoter, and histone octamers cannot bind to it. Each type of state is stable. In order to convert a promoter from the inactive state to the active state, the chromatin structure must be perturbed in order to allow binding of the basal factors.

FIGURE 1. If nucleosomes form at a promoter, transcription factors (and RNA polymerase) cannot bind. If transcription factors (and RNA polymerase) bind to the promoter to establish a stable complex for initiation, histones are excluded.
The general process of inducing changes in chromatin structure is called chromatin remodeling. This consists of mechanisms for repositioning or displacing histones that depend on the input of energy. Many protein–protein and protein–DNA contacts need to be disrupted to release histones from chromatin. There is no free ride: Energy must be provided to disrupt these contacts. FIGURE 2 illustrates the principle of dynamic remodeling by a factor that hydrolyzes ATP. When the histone octamer is released from DNA, other proteins (in this case transcription factors and RNA polymerase) can bind.

FIGURE 2. The dynamic model for transcription of chromatin relies on factors that can use energy provided by hydrolysis of ATP to displace nucleosomes from specific DNA sequences.
Chromatin remodeling results in several alternative outcomes, as shown in FIGURE 3 :
- Histone octamers may slide along DNA, changing the relationship between the nucleic acid and the protein. This can alter both the rotational and the translational position of a particular sequence on the nucleosome.
- The spacing between histone octamers may be changed, again with the result that the positions of individual sequences are altered relative to the histone octamer.
- The most extensive change is that an octamer(s) may be displaced entirely from DNA to generate a nucleosome-free gap. Alternatively, one or both H2A-H2B dimers can be displaced, leaving an H2A-H2B-H3-H4 hexamer, or an H3-H4 tetramer, on the DNA.
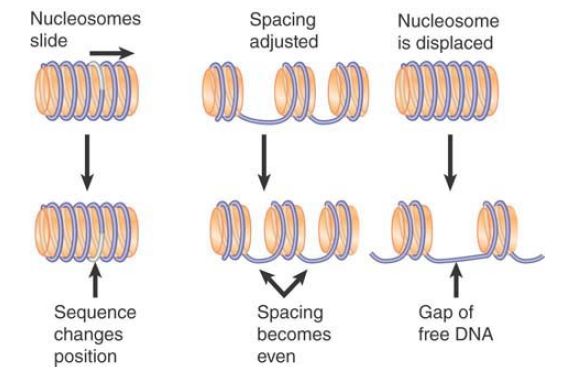
FIGURE 3. Remodeling complexes can cause nucleosomes to slide along DNA, displace nucleosomes from DNA, or reorganize the spacing between nucleosomes.
A major role of chromatin remodeling is to change the organization of nucleosomes at the promoter of a gene that is to be transcribed. This is required to allow the transcription apparatus to gain access to the promoter. Remodeling can also act to prevent transcription by moving nucleosomes onto, rather than away from, essential promoter sequences. Remodeling is also required to enable other manipulations of chromatin, such as repair of damaged DNA .
Remodeling often takes the form of displacing one or more histone octamers. This can result in the creation of a site that is hypersensitive to cleavage with DNase I . Sometimes less dramatic changes are observed, such as alteration of the rotational positioning of a single nucleosome, detectable by loss or change of the DNase I 10-bp ladder. Thus, changes in chromatin structure can extend from subtly altering the positions of nucleosomes to removing them altogether.
Chromatin remodeling is undertaken by ATP-dependent chromatin remodeling complexes, which use ATP hydrolysis to provide the energy for remodeling. The heart of the remodeling complex is its ATPase subunit. The ATPase subunits of all remodeling complexes are related members of a large superfamily of proteins, which is divided into subfamilies of more closely related members. Remodeling complexes are classified according to the subfamily of ATPase that they contain as their catalytic subunit. There are many subfamilies; four major ones (SWI/SNF,ISWI, CHD, and INO80/SWR1) are shown in TABLE .1. The first remodeling complex described was the SWI/SNF (“switch sniff”) complex in yeast, which has homologs in all eukaryotes. The chromatin remodeling superfamily is large and diverse, and most species have multiple complexes in different subfamilies. Budding yeast have two SWI/SNF-related complexes and three ISWI complexes. At least four different ISWI complexes have been characterized in mammals. Remodeling complexes range from small heterodimeric complexes (the ATPase subunit plus a single partner) to massive complexes of 10 or more subunits. Each type of complex may undertake a different range of remodeling activities.
TABLE .1 Remodeling complexes can be classified by their ATPase subunits.
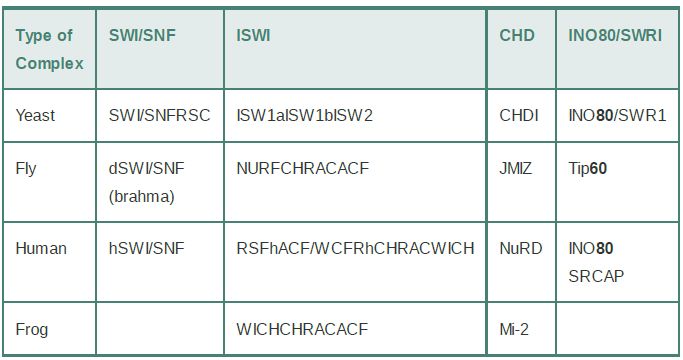
SWI/SNF is the prototypic remodeling complex. Its name reflects the fact that many of its subunits are encoded by genes originally identified by swi or snf mutations in Saccharomyces cerevisiae. (swi mutants cannot switch mating type, and snf—sucrose nonfermenting—mutants cannot use sucrose as a carbon source.)
Mutations in these loci are pleiotropic, and the range of defects is similar to those shown by mutants that have lost part of the CTD of RNA polymerase II. Early hints that these genes might be linked to chromatin came from evidence that these mutations show genetic interactions with mutations in genes that code for components of chromatin: SIN1, which encodes a nonhistone chromatin protein, and SIN2, which encodes histone H3. The SWI and SNF genes are required for expression of a variety of individual loci. Approximately 120 S. cerevisiae genes require SWI/SNF for normal expression, which is about 2% of the total number of genes. Expression of these loci may require the SWI/SNF complex to remodel chromatin at their promoters. Each yeast cell has only about 150 complexes of SWI/SNF. The related RSC (remodels the structure of chromatin) complex is more abundant and is essential for viability. It acts at approximately 700 target loci.
Different subfamilies of remodeling complexes have distinct modes of remodeling, reflecting differences in their ATPase subunits, as well as effects of other proteins in individual remodeling complexes. SWI/SNF complexes can remodel chromatin in vitro without overall loss of histones or can displace histone octamers. These reactions likely pass through the same intermediate in which the structure of the target nucleosome is altered, leading either to reformation of a (remodeled) nucleosome on the original DNA or to displacement of the histone octamer to a different DNA molecule. In contrast, the ISWI family primarily affects nucleosome positioning without displacing octamers, in a sliding reaction in which the octamer moves along DNA. The activity of ISWI requires the histone H4 tail as well as binding to linker DNA.
The DNA and histone octamer have many contact points; 14 have been identified in the crystal structure. All of these contacts must be broken for an octamer to be released or for it to move to a new position. How is this achieved? The ATPase subunits are distantly related to helicases (enzymes that unwind double-stranded nucleic acids), but remodeling complexes do not have any unwinding activity. Present thinking is that remodeling complexes in the SWI/SNF and ISWI classes use the hydrolysis of ATP to translocate DNA on the nucleosomal surface, essentially by creating a twisting motion. This twisting creates a mechanical force that allows a small region of DNA to be released from the surface and then repositioned. This mechanism creates transient loops of DNA on the surface of the octamer; these loops are themselves accessible to interact with other factors, or they can propagate along the nucleosome, ultimately resulting in nucleosome sliding. In the case of SWI/SNF complexes, this activity can also result in nucleosome disassembly, first by displacement of the H2A/H2B dimers, then of the H3/H4 tetramer.
Different remodeling complexes have different roles in the cell. SWI/SNF complexes are frequently involved in transcriptional activation, whereas some ISWI complexes act as repressors, using their remodeling activity to slide nucleosomes onto promoter regions to prevent transcription. Members of the CHD (chromodomain helicase DNA-binding) family have also been implicated in repression, particularly the Mi-2/NuRD complexes, which contain both chromatin remodeling and histone deacetylase activities. Remodelers in the SWR1/INO80 class have a unique activity: In addition to their normal remodeling capabilities, some members of this class also have histone exchange capability, in which individual histones (usually H2A/H2B dimers) can be replaced in a nucleosome, typically with the H2AZ histone variant .
 الاكثر قراءة في مواضيع عامة في الاحياء الجزيئي
الاكثر قراءة في مواضيع عامة في الاحياء الجزيئي
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















