
Catalytic Hydrogenation
 المؤلف:
LibreTexts Project
المؤلف:
LibreTexts Project
 المصدر:
................
المصدر:
................
 الجزء والصفحة:
.................
الجزء والصفحة:
.................
 13-1-2020
13-1-2020
 2155
2155
Catalytic Hydrogenation of Alkenes
Addition of hydrogen to a carbon-carbon double bond is called hydrogenation. The overall effect of such an addition is the reductive removal of the double bond functional group. Regioselectivity is not an issue, since the same group (a hydrogen atom) is bonded to each of the double bond carbons. The simplest source of two hydrogen atoms is molecular hydrogen (H2), but mixing alkenes with hydrogen does not result in any discernible reaction. Although the overall hydrogenation reaction is exothermic, a high activation energy prevents it from taking place under normal conditions. This restriction may be circumvented by the use of a catalyst, as shown in the following diagram.
An example of an alkene addition reaction is a process called hydrogenation.In a hydrogenation reaction, two hydrogen atoms are added across the double bond of an alkene, resulting in a saturated alkane. Hydrogenation of a double bond is a thermodynamically favorable reaction because it forms a more stable (lower energy) product. In other words, the energy of the product is lower than the energy of the reactant; thus it is exothermic (heat is released). The heat released is called the heat of hydrogenation, which is an indicator of a molecule’s stability.
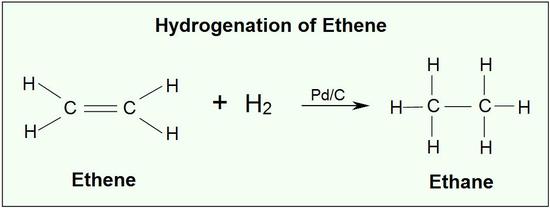
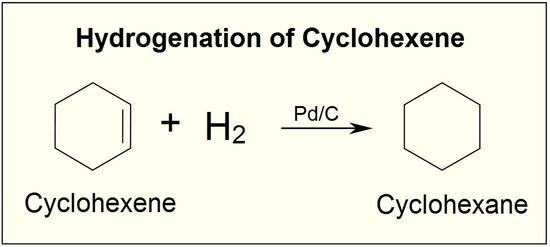
Catalysts are substances that changes the rate (velocity) of a chemical reaction without being consumed or appearing as part of the product. Catalysts act by lowering the activation energy of reactions, but they do not change the relative potential energy of the reactants and products. Finely divided metals, such as platinum, palladium and nickel, are among the most widely used hydrogenation catalysts. Catalytic hydrogenation takes place in at least two stages, as depicted in the diagram. First, the alkene must be adsorbed on the surface of the catalyst along with some of the hydrogen. Next, two hydrogens shift from the metal surface to the carbons of the double bond, and the resulting saturated hydrocarbon, which is more weakly adsorbed, leaves the catalyst surface. The exact nature and timing of the last events is not well understood.
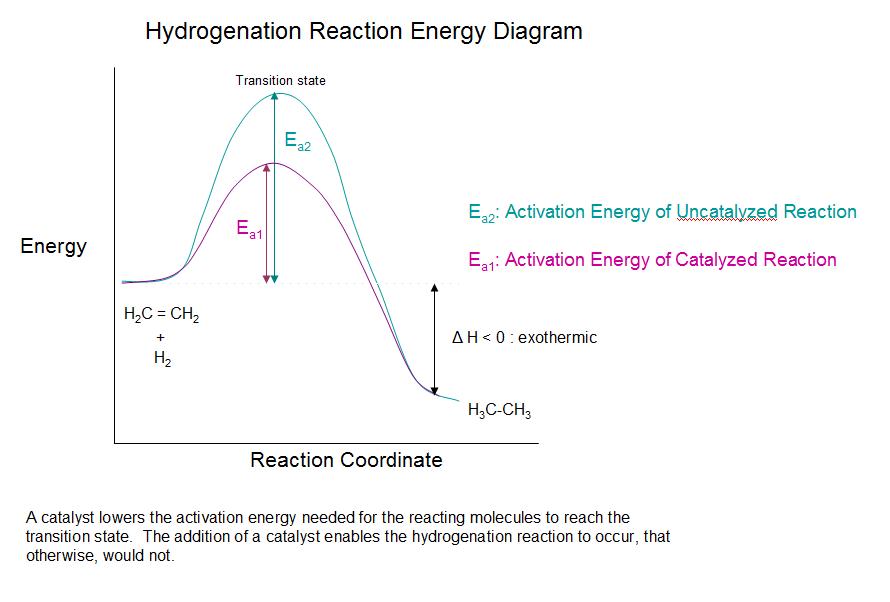
As shown in the energy diagram, the hydrogenation of alkenes is exothermic, and heat is released corresponding to the ΔE (colored green) in the diagram. This heat of reaction can be used to evaluate the thermodynamic stability of alkenes having different numbers of alkyl substituents on the double bond. For example, the following table lists the heats of hydrogenation for three C5H10 alkenes which give the same alkane product (2-methylbutane). Since a large heat of reaction indicates a high energy reactant, these heats are inversely proportional to the stabilities of the alkene isomers. To a rough approximation, we see that each alkyl substituent on a double bond stabilizes this functional group by a bit more than 1 kcal/mole.
| Alkene Isomer |
(CH3)2CHCH=CH2
3-methyl-1-butene |
CH2=C(CH3)CH2CH3
2-methyl-1-butene |
(CH3)2C=CHCH3
2-methyl-2-butene |
Heat of Reaction
( ΔHº ) |
–30.3 kcal/mole |
–28.5 kcal/mole |
–26.9 kcal/mole |
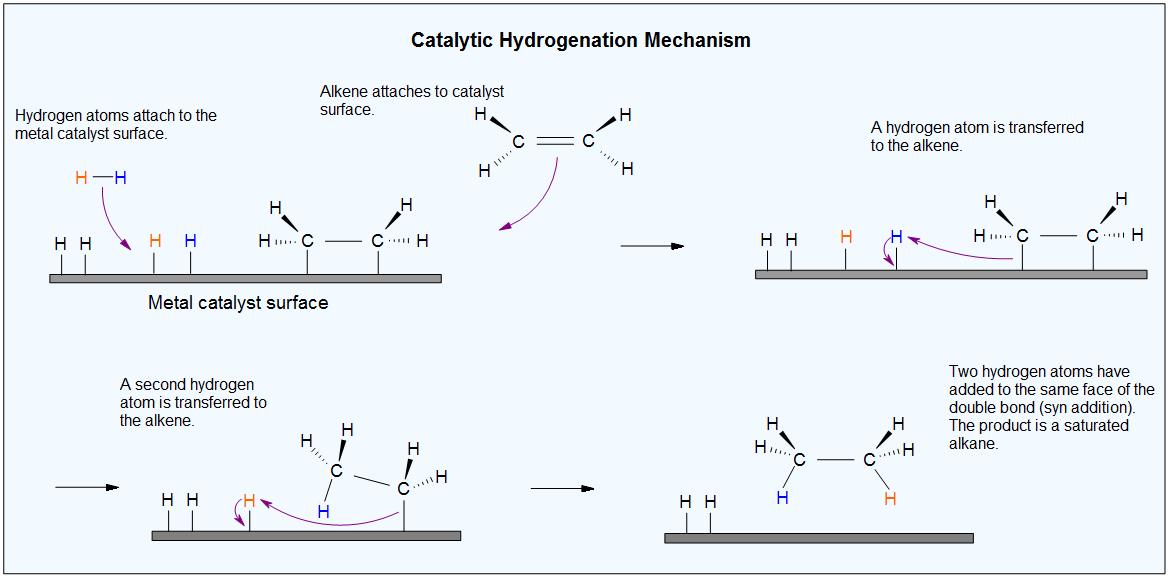
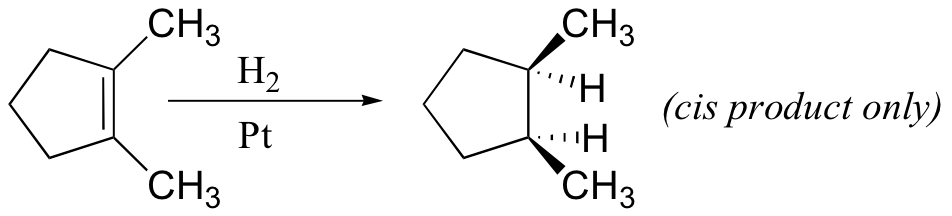
From the mechanism shown here we would expect the addition of hydrogen to occur with syn-stereoselectivity. This is often true, but the hydrogenation catalysts may also cause isomerization of the double bond prior to hydrogen addition, in which case stereoselectivity may be uncertain.
 الاكثر قراءة في الهايدروكاربونات
الاكثر قراءة في الهايدروكاربونات
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


