
Hydrogen Bonding
 المؤلف:
William Reusch
المؤلف:
William Reusch
 المصدر:
Virtual Textbook of Organic Chemistry
المصدر:
Virtual Textbook of Organic Chemistry
 الجزء والصفحة:
............
الجزء والصفحة:
............
 12-7-2018
12-7-2018
 2155
2155
Hydrogen Bonding
The most powerful intermolecular force influencing neutral (uncharged) molecules is the hydrogen bond. If we compare the boiling points of methane (CH4) -161ºC, ammonia (NH3) -33ºC, water (H2O) 100ºC and hydrogen fluoride (HF) 19ºC, we see a greater variation for these similar sized molecules than expected from the data presented above for polar compounds. This is shown graphically in the following chart. Most of the simple hydrides of group IV, V, VI & VII elements display the expected rise in boiling point with molecular mass, but the hydrides of the most electronegative elements (nitrogen, oxygen and fluorine) have abnormally high boiling points for their mass.
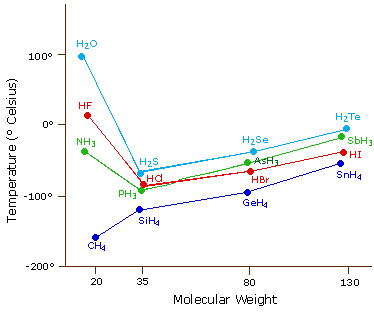
The exceptionally strong dipole-dipole attractions that cause this behavior are called the hydrogen bond. Hydrogen forms polar covalent bonds to more electronegative atoms such as oxygen, and because a hydrogen atom is quite small, the positive end of the bond dipole (the hydrogen) can approach neighboring nucleophilic or basic sites more closely than can other polar bonds. Coulombic forces are inversely proportional to the sixth power of the distance between dipoles, making these interactions relatively strong, although they are still weak (ca. 4 to 5 kcal per mole) compared with most covalent bonds. The unique properties of water are largely due to the strong hydrogen bonding that occurs between its molecules. In the following diagram the hydrogen bonds are depicted as magenta dashed lines.
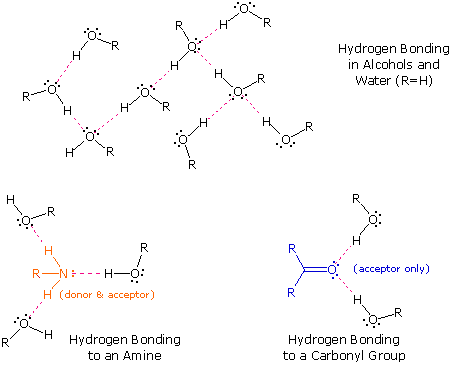
The molecule providing a polar hydrogen for a hydrogen bond is called a donor. The molecule that provides the electron rich site to which the hydrogen is attracted is called an acceptor. Water and alcohols may serve as both donors and acceptors, whereas ethers, aldehydes, ketones and esters can function only as acceptors. Similarly, primary and secondary amines are both donors and acceptors, but tertiary amines function only as acceptors. Once you are able to recognize compounds that can exhibit intermolecular hydrogen bonding, the relatively high boiling points they exhibit become understandable. The data in the following table serve to illustrate this point.
|
Compound
|
Formula
|
Mol. Wt.
|
Boiling Point
|
Melting Point
|
|
dimethyl ether
|
CH3OCH3
|
46
|
–24ºC
|
–138ºC
|
|
ethanol
|
CH3CH2OH
|
46
|
78ºC
|
–130ºC
|
|
propanol
|
CH3(CH2)2OH
|
60
|
98ºC
|
–127ºC
|
|
diethyl ether
|
(CH3CH2)2O
|
74
|
34ºC
|
–116ºC
|
|
propyl amine
|
CH3(CH2)2NH2
|
59
|
48ºC
|
–83ºC
|
|
methylaminoethane
|
CH3CH2NHCH3
|
59
|
37ºC
|
|
|
trimethylamine
|
(CH3)3N
|
59
|
3ºC
|
–117ºC
|
|
ethylene glycol
|
HOCH2CH2OH
|
62
|
197ºC
|
–13ºC
|
|
acetic acid
|
CH3CO2H
|
60
|
118ºC
|
17ºC
|
|
ethylene diamine
|
H2NCH2CH2NH2
|
60
|
118ºC
|
8.5ºC
|
Alcohols boil considerably higher than comparably sized ethers (first two entries), and isomeric 1º, 2º & 3º-amines, respectively, show decreasing boiling points, with the two hydrogen bonding isomers being substantially higher boiling than the 3º-amine (entries 5 to 7). Also, O–H---O hydrogen bonds are clearly stronger than N–H---N hydrogen bonds, as we see by comparing propanol with the amines. As expected, the presence of two hydrogen bonding functions in a compound raises the boiling point even further. Acetic acid (the ninth entry) is an interesting case.
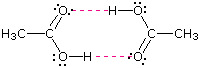
A dimeric species, shown on the right, held together by two hydrogen bonds is a major component of the liquid state. If this is an accurate representation of the composition of this compound then we would expect its boiling point to be equivalent to that of a C4H8O4 compound (formula weight = 120). A suitable approximation of such a compound is found in tetramethoxymethane, (CH3O)4C, which is actually a bit larger (formula weight = 136) and has a boiling point of 114ºC. Thus, the dimeric hydrogen bonded structure appears to be a good representation of acetic acid in the condensed state.
A related principle is worth noting at this point. Although the hydrogen bond is relatively weak (ca. 4 to 5 kcal per mole), when several such bonds exist the resulting structure can be quite robust. The hydrogen bonds between cellulose fibers confer great strength to wood and related materials.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


