

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Complex ions of group 1 metals
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المصدر:
Inorganic Chemistry
الجزء والصفحة:
p 268
16-1-2018
1726
Complex ions of group 1 metals
Unlike simple inorganic ligands, polyethers and, in particular, cyclic polyethers complex alkali metal ions quite strongly. The crown ethers are cyclic ethers which include 1,4,7,10,13,16-hexaoxacyclooctadecane (Figure 1.1a), the common name for which is 18-crown-6; this nomenclature gives the total number (C + O) and number of O atoms in the ring. Figure 1.1b shows the structure of the [K(18- crown-6)]+ cation; the K ion is coordinated by the six Odonors.

The radius of the cavity† inside the 18-crown-6 ring is 140 pm, and this compares with values of rion for the alkali metal ions ranging from 76pm for Li to 170pm for Cs (Table 1.1).
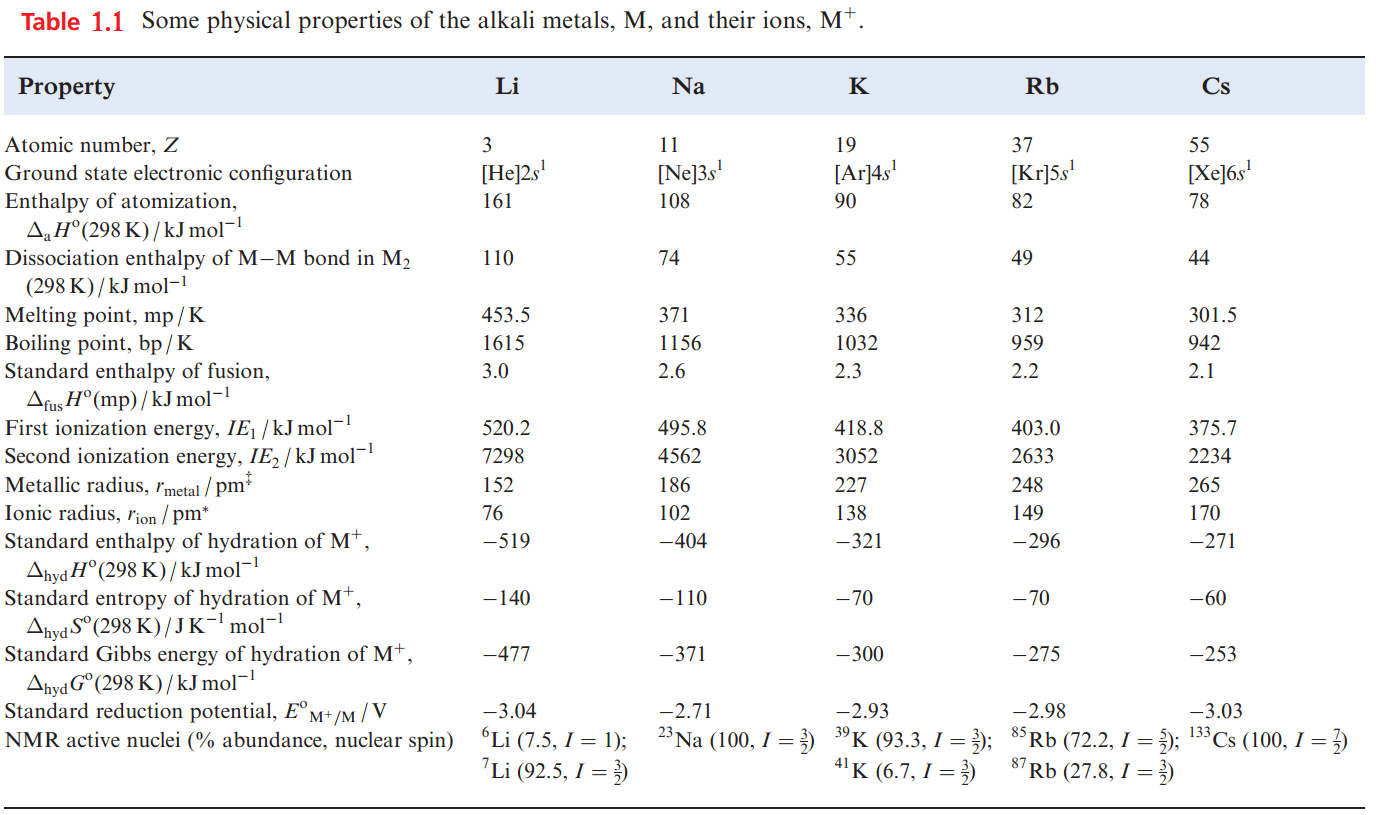
The radius of the K ion (138 pm) is well matched to that of the macrocycle, and stability constants for the formation of [M(18-crown-6)] in acetone follow the sequence K+> Rb+> Cs+ ≈ Na+> Li+.
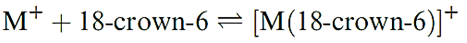
Different crown ethers have different cavity sizes, although the latter is not a fixed property because of the ability of the ligand to change conformation. Thus, the radii of the holes in 18-crown-6, 15-crown-5 and 12-crown-4 can be taken to be roughly 140, 90 and 60pm respectively. It is, however, dangerous to assume that an [ML]+ complex will fail to form simply because the size of M+ is not matched correctly to the hole size of the macrocyclic ligand L. For example, if the radius of M+ is slightly larger than the radius of L, a complex may form in which M+ sits above the plane containing the donor atoms, e.g. [Li(12-crown-4)Cl] .
Alternatively a 1 :2 complex [ML2] + may result in which the metal ion is sandwiched between two ligands, e.g. [Li(12-crown-4)2] +. Note that these latter examples refer to complexes crystallized from solution.
 الاكثر قراءة في الجدول الدوري وخواص العناصر
الاكثر قراءة في الجدول الدوري وخواص العناصر
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















