
The hydrogen bond
 المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
المؤلف:
CATHERINE E. HOUSECROFT AND ALAN G. SHARPE
 المصدر:
Inorganic Chemistry
المصدر:
Inorganic Chemistry
 الجزء والصفحة:
p 244
الجزء والصفحة:
p 244
 5-1-2018
5-1-2018
 3782
3782
The hydrogen bond
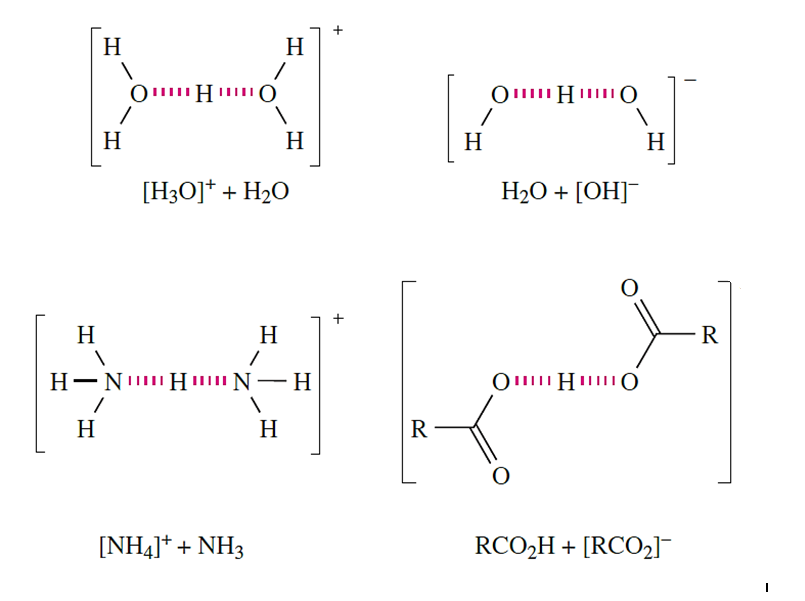
A hydrogen bond is formed between anHatom attached to an electronegative atom, and an electronegative atom that possesses a lone pair of electrons. Physical and solid state structural data for many compounds provide evidence for the formation of intermolecular hydrogen bonds. Such interactions arise between an H atom attached to an electronegative atom, and an electronegative atom bearing a lone pair of electrons, i.e. X_H……Y where atom Y may or may not be the same as X. It is not necessary for the electronegative atom X to be highly electronegative for there to be a meaningful hydrogen- bonded interaction. Thus, in addition to hydrogen bonds of the type F_H……F, O_H……F, N_H……F, O_H……O, N_H……O, O_H……N and N_H……N, it is now well recognized that weaker hydrogen bonds, in particular C_H……O interactions, play an important role in the solid state structures of small molecules and biological systems. The wide variety of interactions that are now classed as hydrogen bonds means that the definition of the latter must not be too restrictive. A modern definition of a hydrogen bond which does not rely directly on the concept of electronegativity has been proposed by Steiner:
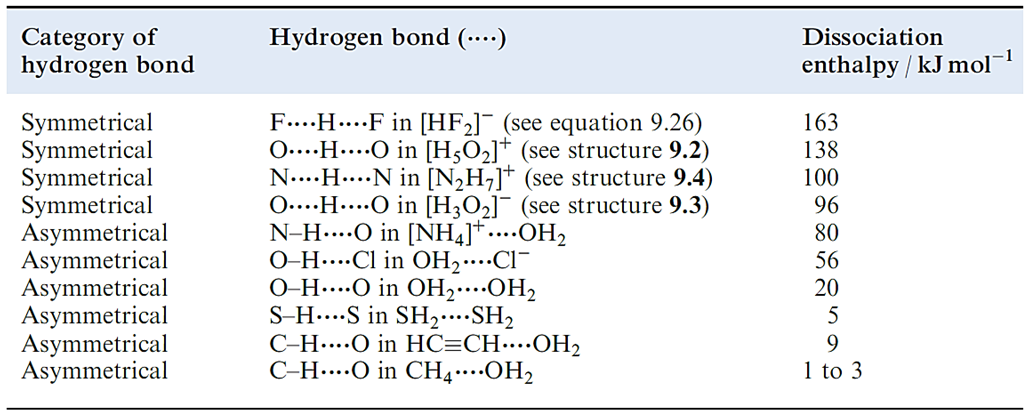
An X_H……Y interaction is called a hydrogen bond if it constitutes a local bond, and if X_H acts as a proton donor to Y. It is now well recognized that the term ‘hydrogen bonding’ covers a wide range of interactions with a corresponding variation in strengths of interaction. Table 1.1 lists representative examples.
We have already described the hydrogen-bonded network in ice Here, as in most hydrogen-bonded interactions, the H atom is asymmetrically positioned with respect to the two atoms with which it interacts. Association in carboxylic acids is a consequence of hydrogen bonding. In a typical X_H……Y interaction, the X_H covalent bond is slightly longer and weaker than a comparable bond in the absence of hydrogen bonding. In such cases, the interaction may be considered in terms of an electrostatic interaction between a covalently bonded H with a δ+ charge, and a lone pair of electrons on the adjacent atom. Some experimental observations cannot be rationalized within a purely electrostatic model, and point towards a covalent contribution, the importance of which increases as the hydrogen bond becomes stronger. Table 1.1 shows typical values of bond dissociation enthalpies of some hydrogen bonds. The data in the table have been obtained from calculations on isolated species. These enthalpy values are therefore only approximate when applied to hydrogen bonds between molecules in a solid state lattice; enthalpy values for these interactions cannot be measured directly. An example of how the strengths of hydrogen bonds can be obtained experimentally comes from the dissociation of a carboxylic acid dimer in the vapour state.

Table 1.1 Typical values for the enthalpies of dissociation of different types of hydrogen bonds. Values are calculated for gas-phase species
The position of above equilibrium is temperature-dependent, and ΔHo for the reaction can be obtained from the variation of Kp with temperature:
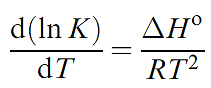
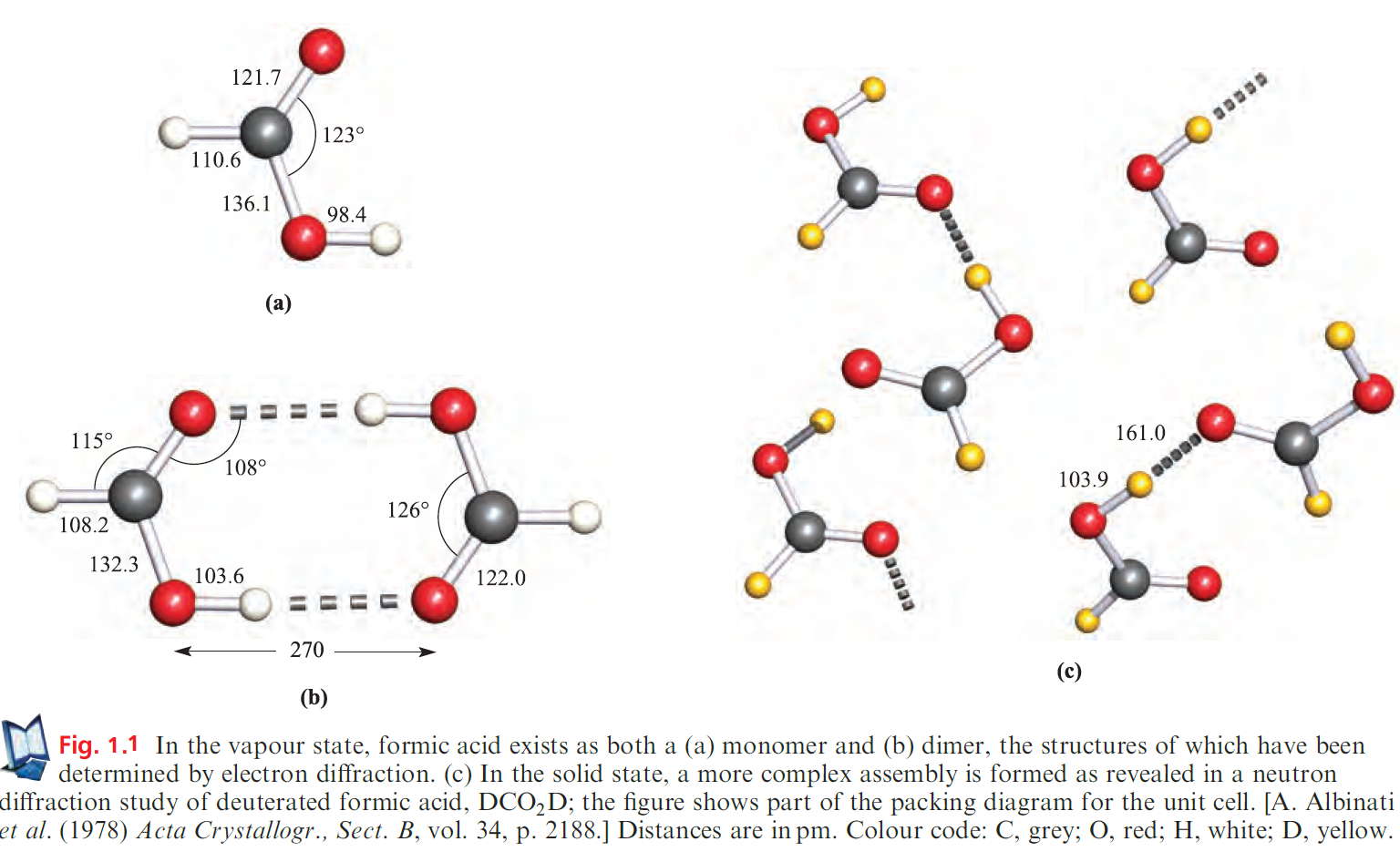
For formic acid (methanoic acid),ΔHo for the dissociation in equation 9.25 is found to be 60 kJ mol-1, or the value can be expressed as 30 kJ per mole of hydrogen bonds. This quantity is often referred to as the hydrogen-bond energy, but this is not strictly correct since other bonds change slightly when hydrogen bonds are broken (Figures 1.1a and 1.1b).
In some hydrogen-bonded interactions, the H atom is symmetrically positioned, e.g. in [HF2]- or [H5O2]+. In the formation of [HF2]- appreciable stretching of the original covalent H_F bond takes place, to give two equivalent H……F interactions.
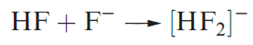
The bonding in symmetrical X….H…..X interactions is best considered in terms of a 3c-2e interaction, i.e. as a delocalized interaction such as was described for B2H6 . Each H……F bond is relatively strong (Table 1.1), with the bond dissociation enthalpy being of a similar magnitude to that of the F_F bond in F2 (158 kJ mol-1); compare this with the bond dissociation enthalpy of HF (570 kJ mol-1). Strong, symmetrical hydrogen bonds with covalent character usually occur between like atoms (see Table 1.1). Common examples involve interactions between an acid and its conjugate base where there is no distinction between the donor (X) and acceptor (Y) atoms.
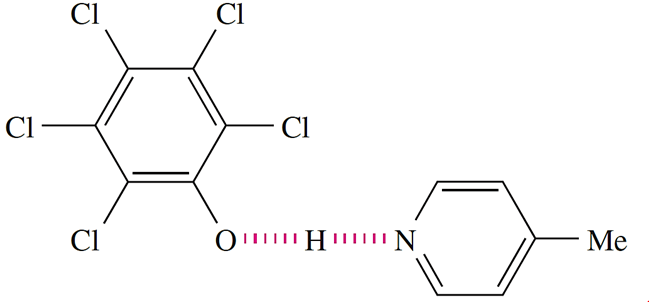
Neutron diffraction studies have confirmed that adduct 9.6 contains a strong, symmetrical N……H…..O hydrogen bond at 90K (O_H = N_H =126 pm). However, the system is complicated by the observation that the H atom migrates towards the O atom as the temperature is lowered from 200 to 20 K.†
The use of the qualitative descriptors ‘strong’, ‘moderate’ (or ‘normal’) and ‘weak’ for hydrogen bonds is common. For example, strong O….H…..O interactions are typified by O……O separations close to 240 pm, while moderate O_H……O interactions are characterized by longer O…..O distances, up to -280 pm. Accurate neutron and X-ray diffraction data‡ confirm that for O_H…..O interactions, shortening of the O………..O distance from 280 to 240pm is accompanied by a change from asymmetrical, electrostatic = hydrogen bonds to symmetrical, covalent interactions.
Strong hydrogen bonds are usually linear (i.e. the X_H_Y angle is close to 1808), while in ‘moderate’ hydrogen bonds, X_H_Y angles may range from 1308 to 1808. Thetransition from ‘strong’ to ‘moderate’ hydrogen bonds is not clear-cut.
 الاكثر قراءة في الجدول الدوري وخواص العناصر
الاكثر قراءة في الجدول الدوري وخواص العناصر
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


