

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Industrial Preparation and Use of Alkenes
المؤلف:
John McMurry
المصدر:
Organic Chemistry
الجزء والصفحة:
9th. p 186
16-5-2017
2451
Industrial Preparation and Use of Alkenes
Ethylene and propylene, the simplest alkenes, are the two most important organic chemicals produced industrially. Approximately 127 million metric tons of ethylene and 54 million metric tons of propylene are produced worldwide each year for use in the synthesis of polyethylene, polypropylene, ethylene glycol, acetic acid, acetaldehyde, and a host of other substances (Figure1.1).
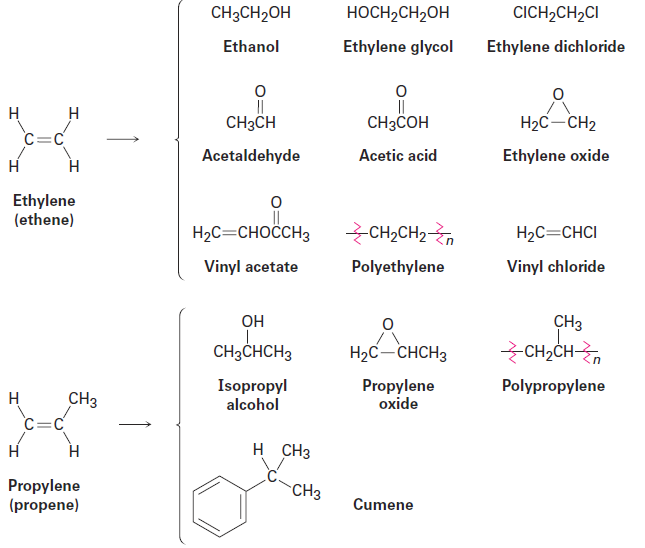
Figure1.1 Compounds derived industrially from ethylene and propylene.
Ethylene, propylene, and butene are synthesized industrially by steam cracking of light (C2–C8) alkanes.

Steam cracking takes place without a catalyst at temperatures up to 900 °C. The process is complex, although it undoubtedly involves radical reactions. The high-temperature reaction conditions cause spontaneous homolytic breaking of C-C and C-H bonds, with resultant formation of smaller fragments. We might imagine, for instance, that a molecule of butane splits into two ethyl radicals, each of which then loses a hydrogen atom to generate two molecules of ethylene.
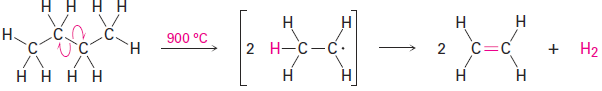
Steam cracking is an example of a reaction whose energetics are dominated by entropy (ΔS°) rather than by enthalpy (ΔH°) in the free-energy equation ΔG° = ΔH° - TΔS°. Although the bond dissociation energy Δ for a carbon–carbon single bond is relatively high (about 370 kJ/mol) and cracking is endothermic, the large positive entropy change resulting from the fragmentation of one large molecule into several smaller pieces, together with the high temperature, makes the TΔS° term larger than the ΔH° term, thereby favoring the cracking reaction.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















