


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 3-9-2020
Date: 31-8-2020
Date: 28-1-2019
|
Chain reactions and critical mass
Take a look at the equation for the fission of uranium-235 (U-235):
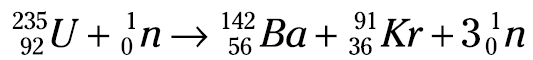
Notice that one neutron is used, but three are produced. These three neutrons, if they encounter other U-235 atoms, can initiate other fissions, producing even more neutrons. It’s the old domino effect — or in terms of nuclear chemistry, it’s a continuing cascade of nuclear fissions called a chain reaction. Figure 1.1 shows the chain reaction of U-235.
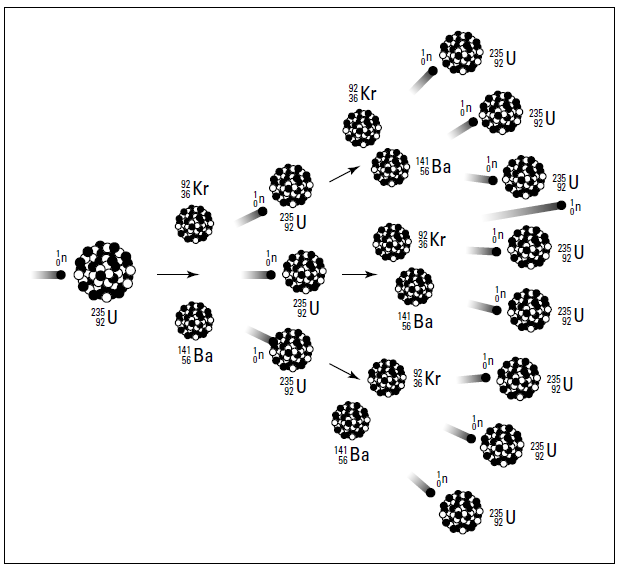
Figure 1.1: Chain reaction.
A chain reaction depends on the release of more neutrons than are used during the nuclear reaction. If you were to write the equation for the nuclear fission of U-238, the more abundant isotope of uranium, you’d use one neutron and get only one back out. So you can’t have a chain reaction with U-238.
But isotopes that produce an excess of neutrons in their fission support a chain reaction. This type of isotope is said to be fissionable, and only two main fissionable isotopes are used during nuclear reactions: U-235 and plutonium-239 (Pu-239). Critical mass is the minimum amount of fissionable matter you need to support a self-sustaining chain reaction. That amount related to those neutrons. If the sample is small, then the neutrons are likely to shoot out of the sample before hitting a U-235 nucleus. If they don’t hit a U-235 nucleus, no extra electrons and no energy are released. The reaction just fizzles. Anything less than the critical mass is called subcritical.



|
|
|
|
تحذير من "عادة" خلال تنظيف اللسان.. خطيرة على القلب
|
|
|
|
|
|
|
دراسة علمية تحذر من علاقات حب "اصطناعية" ؟!
|
|
|
|
|
|
|
العتبة العباسية المقدسة تحذّر من خطورة الحرب الثقافية والأخلاقية التي تستهدف المجتمع الإسلاميّ
|
|
|