


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 26-9-2021
Date: 26-8-2021
Date: 10-9-2021
|
Cholesterol Synthesis Regulation
HMG CoA reductase is the major control point for cholesterol biosynthesis and is subject to different kinds of metabolic control.
1. Sterol-dependent regulation of gene expression: Expression of the gene for HMG CoA reductase is controlled by the trans-acting factor, sterol regulatory element–binding protein-2 (SREBP-2), which binds DNA at the cis-acting sterol regulatory element (SRE) upstream of the reductase gene. Inactive SREBP-2 is an integral protein of the SER membrane and associates with a second SER membrane protein, SREBP cleavage–activating protein (SCAP). When sterol levels in the SER are low, the SREBP-2–SCAP complex moves from the ER to the Golgi.
In the Golgi membrane, SREBP-2 is sequentially acted upon by two proteases, which generate a soluble fragment that enters the nucleus, binds the SRE, and functions as a transcription factor. This results in increased synthesis of HMG CoA reductase and, therefore, increased cholesterol synthesis (Fig.1). However, if sterols are abundant, they bind SCAP at its sterolsensing domain and induce the binding of SCAP to yet other ER membrane proteins, the insulin-induced gene proteins (INSIG). This results in the retention of the SCAP–SREBP complex in the SER, thereby preventing the activation of SREBP-2 and leading to downregulation of cholesterol synthesis. [Note: SREBP-1c upregulates expression of enzymes involved in FA synthesis in response to insulin .]
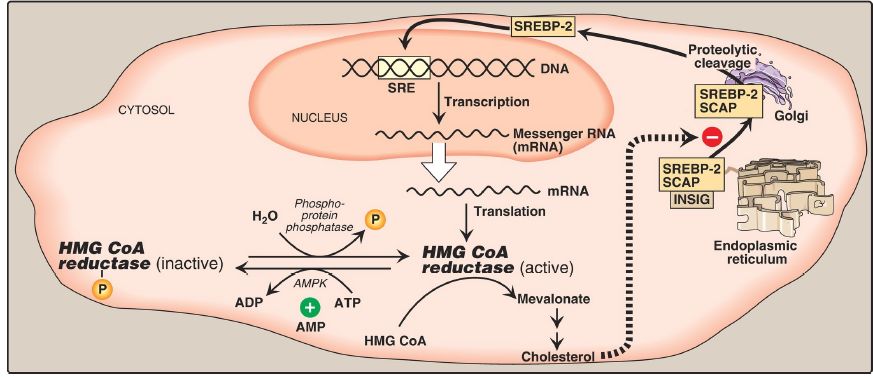
Figure 1: Regulation of hydroxymethylglutaryl coenzyme A (HMG CoA) reductase. SRE = sterol regulatory element; SREBP = SRE-binding protein; SCAP = SREBP cleavage–activating protein; AMPK = adenosine monophosphate–activated protein kinase; ADP = adenosine diphosphate; = phosphate; INSIG = insulin-induced gene protein.
2. Sterol-accelerated enzyme degradation: The reductase itself is a sterolsensingintegral protein of the SER membrane. When sterol levels in the SER are high, the enzyme binds to INSIG proteins. Binding leads to cytosolic transfer, ubiquitination, and proteasomal degradation of the reductase .
3. Sterol-independent phosphorylation/dephosphorylation: HMG CoA reductase activity is controlled covalently through the actions of adenosine monophosphate (AMP)–activated protein kinase ([AMPK] ) and a phosphoprotein phosphatase (see Fig. 1). The phosphorylated form of the enzyme is inactive, whereas the dephosphorylated form is active. [Note: Because AMPK is activated by AMP, cholesterol synthesis, like FA synthesis, is decreased when ATP availability is decreased.]
4. Hormonal regulation: The activity of HMG CoA reductase is controlled hormonally. An increase in insulin favors dephosphorylation (activation) of the reductase, whereas an increase in glucagon and epinephrine has the opposite effect.
5. Drug inhibition: The statin drugs (atorvastatin, fluvastatin, lovastatin, pravastatin, rosuvastatin, and simvastatin) are structural analogs of HMG CoA and are (or are metabolized to) reversible, competitive inhibitors of HMG CoA reductase (Fig. 2). They are used to decrease plasma cholesterol levels in patients with hypercholesterolemia.
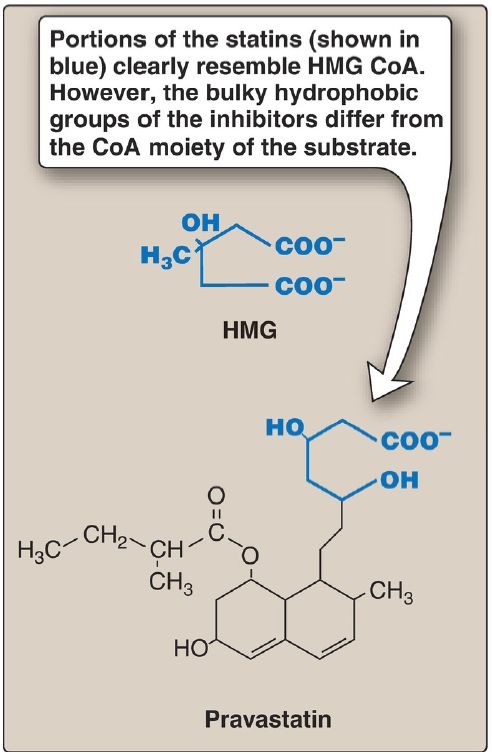
Figure 2: Structural similarity of hydroxymethylglutaric acid (HMG) and pravastatin, a clinically useful cholesterol-lowering drug of the statin family. CoA = coenzyme A.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
المجمع العلمي ينظّم ندوة حوارية حول مفهوم العولمة الرقمية في بابل
|
|
|