


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 6-11-2015
Date: 8-12-2020
Date: 2025-04-01
|
Antibody Structure and Function
Antibodies are Y-shaped dichain glycoproteins of molecular mass approximately 150 kDa (Figure ). They consist of two heavy chains and two light chains, with each chain consisting of two or more domainstha t share the immunoglobulin fold. Each domain is a pleated beta sheet with an intramolecular disulfide bond. The light chain consists of two domains, a light chain variable domain (VL) and a light chain constant domain (CL). There are two types of light chains, kappa and lambda. In humans, approximately two-thirds of light chains are kappa and onethird are lambda. The heavy chain consists of four to five domains, depending on the antibody isotype, and includes a heavy chain variable domain (VH) and three to four constant domains (CH1, CH2, CH3 and CH4). There are a number of different isotypes of antibodies, which include IgG, IgD, IgA, IgM and IgE. The major isotype present in the circulation is IgG, which is also the isotype most frequently used for therapeutic antibodies. The light chains are disulfide linked to the VH–CH1 domains to make the antigen binding fragment (Fab). The two
Fabs are linked via the hinge region to the Fc domain, which consists of CH2–CH3 or CH2–CH4 domains. The Fc region is involved in the elicitation of antibody effector functions including the ability of antibody to mediate cellular killing by immune effector cells [antibody-dependent cellular cytotoxicity (ADCC)] or complement [antibody-dependent cellular cytotoxicity (CDC)].
The smallest antibody fragment capable of binding antigen is the fragment variable or Fv. The Fv consists of the VH and VL domains (Figure ). Within each of the V domains are three complementaritydetermining regions (CDRs), which form loops that comprise the antigen binding region of the V domains. CDRs were first identified by Kabat and Wu as regions with the greatest sequence diversity when different V domains were compared to each other and are also called the hypervariable regions. The six CDRs contribute the majority of the amino acid side-chains that make direct contact with antigen. Each CDR is flanked by regions of more conserved sequence that make up the variable region framework regions. The frameworks fold into the b-strands that comprise the two b-sheets of each V domain and which support the CDRs.
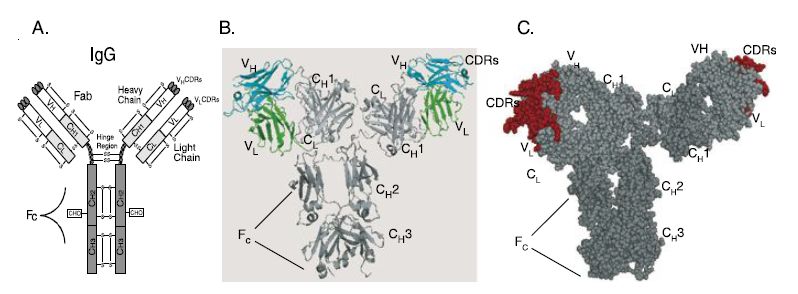
Figure : Structure of IgG antibody and chimeric and humanized antibodies. (A) IgG structure. IgG antibody consists of a pair of heavy and light chains. Each chain consists of the antigen binding variable domains (VH and VL) and one or more constant domains (CH1, CH2, CH3 and CL). Each V or C domain contains an intramolecular disulfide bond (S–S). A single glycosylation site (CHO) exists in the CH2 domain of the heavy chain. The Fab consists of the light chain and the VH–CH1 domains; each IgG consists of two Fab arms. The Fv is the minimal antigen binding unit, consisting of the VH and VL domains. The VH and VL domains contact antigen via the amino acids in the complementarity determining regions (CDRs). The Fc region elicits antibody effector functions such as ADCC and CDC. (B) Alpha carbon backbone tracing of a chimeric antibody. The alpha carbon backbone tracing of a chimeric IgG antibody is shown. The chimeric antibody consists of murine VH and VL domains (colored cyan and green, respectively) and human constant domains (colored shades of gray). (C) Space filling model of a humanized antibody. Murine CDRs in each of the Fab arms are colored red. The framework regions of the V domains and all of the C-domains are colored gray.



|
|
|
|
حقن الذهب في العين.. تقنية جديدة للحفاظ على البصر ؟!
|
|
|
|
|
|
|
علي بابا تطلق نماذج "Qwen" الجديدة في أحدث اختراق صيني لمجال الذكاء الاصطناعي مفتوح المصدر
|
|
|
|
|
|
|
مشاتل الكفيل تنتج أنواعًا مختلفة من النباتات المحلية والمستوردة وتواصل دعمها للمجتمع
|
|
|