


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 5-12-2019
Date: 8-12-2019
Date: 20-7-2018
|
Sucrose, probably the largest-selling pure organic compound in the world, is known as beet sugar, cane sugar, table sugar, or simply sugar. Most of the sucrose sold commercially is obtained from sugar cane and sugar beets (whose juices are 14%–20% sucrose) by evaporation of the water and recrystallization. The dark brown liquid that remains after the recrystallization of sugar is sold as molasses.
The sucrose molecule is unique among the common disaccharides in having an α-1,β-2-glycosidic (head-to-head) linkage. Because this glycosidic linkage is formed by the OH group on the anomeric carbon of α-D-glucose and the OH group on the anomeric carbon of β-D-fructose, it ties up the anomeric carbons of both glucose and fructose.
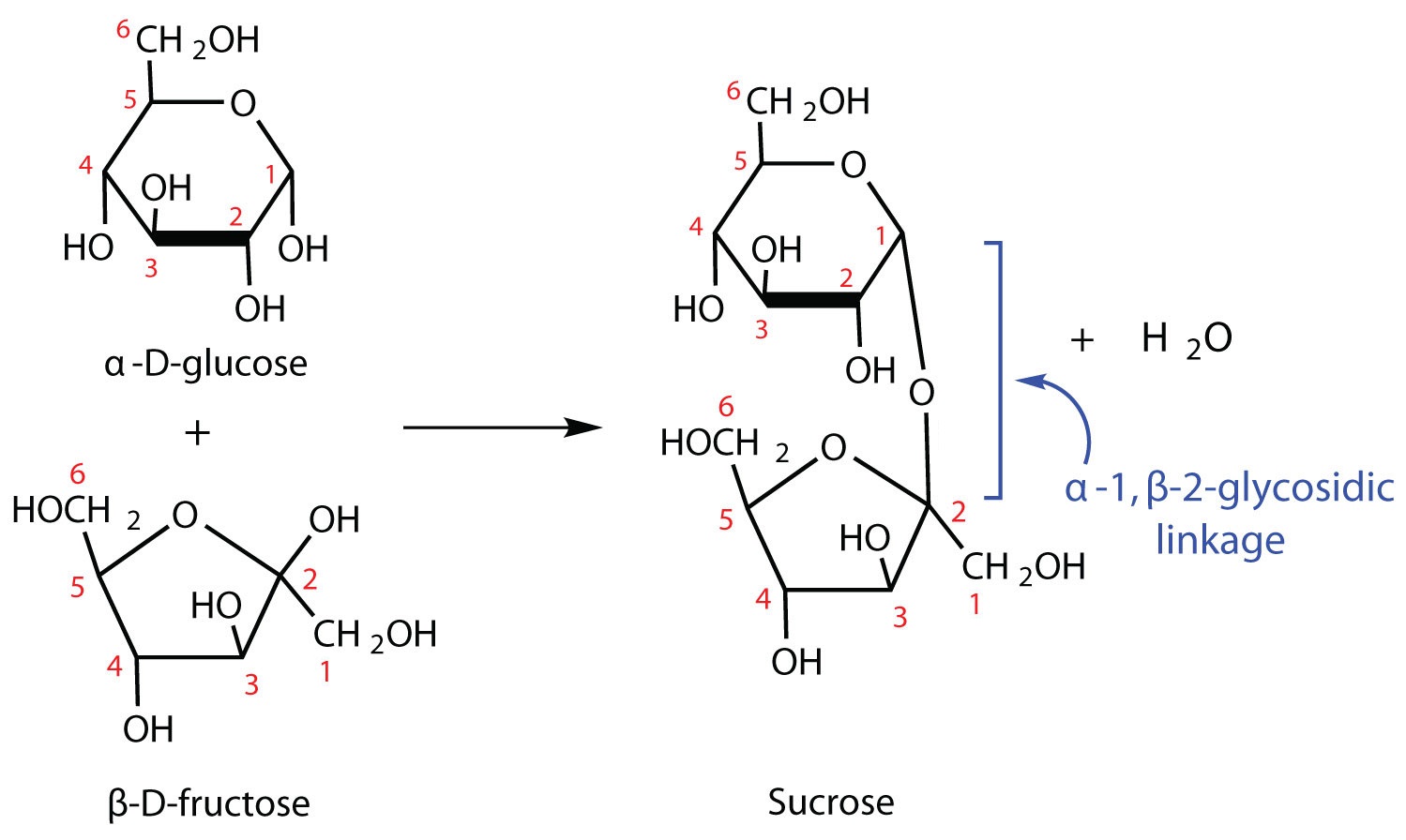
This linkage gives sucrose certain properties that are quite different from those of maltose and lactose. As long as the sucrose molecule remains intact, neither monosaccharide “uncyclizes” to form an open-chain structure. Thus, sucrose is incapable of mutarotation and exists in only one form both in the solid state and in solution. In addition, sucrose does not undergo reactions that are typical of aldehydes and ketones. Therefore, sucrose is a nonreducing sugar.
The hydrolysis of sucrose in dilute acid or through the action of the enzyme sucrase (also known as invertase) gives an equimolar mixture of glucose and fructose. This 1:1 mixture is referred to as invert sugar because it rotates plane-polarized light in the opposite direction than sucrose. The hydrolysis reaction has several practical applications. Sucrose readily recrystallizes from a solution, but invert sugar has a much greater tendency to remain in solution. In the manufacture of jelly and candy and in the canning of fruit, the recrystallization of sugar is undesirable. Therefore, conditions leading to the hydrolysis of sucrose are employed in these processes. Moreover, because fructose is sweeter than sucrose, the hydrolysis adds to the sweetening effect. Bees carry out this reaction when they make honey.
The average American consumes more than 100 lb of sucrose every year. About two-thirds of this amount is ingested in soft drinks, presweetened cereals, and other highly processed foods. The widespread use of sucrose is a contributing factor to obesity and tooth decay. Carbohydrates such as sucrose, are converted to fat when the caloric intake exceeds the body’s requirements, and sucrose causes tooth decay by promoting the formation of plaque that sticks to teeth.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
المجمع العلمي ينظّم ندوة حوارية حول مفهوم العولمة الرقمية في بابل
|
|
|