


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 15-12-2016
Date: 28-6-2019
Date: 28-7-2018
|
The carboxyl group is associated with two characteristic infrared stretching absorptions which change markedly with hydrogen bonding. The spectrum of a CCl4 solution of propionic acid (propanoic acid), shown below, is illustrative. Carboxylic acids exist predominantly as hydrogen bonded dimers in condensed phases. The O-H stretching absorption for such dimers is very strong and broad, extending from 2500 to 3300 cm-1. This absorption overlaps the sharper C-H stretching peaks, which may be seen extending beyond the O-H envelope at 2990, 2950 and 2870 cm-1. The smaller peaks protruding near 2655 and 2560 are characteristic of the dimer. In ether solvents a sharper hydrogen bonded monomer absorption near 3500 cm-1 is observed, due to competition of the ether oxygen as a hydrogen bond acceptor. The carbonyl stretching frequency of the dimer is found near 1710 cm-1, but is increased by 25 cm-1 or more in the monomeric state. Other characteristic stretching and bending absorptions are marked in the spectrum.
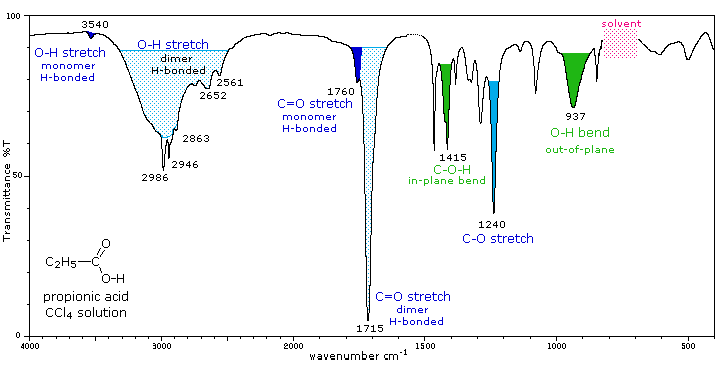 |
Nitriles show a distinctive absorption for the C-N triple bond which appear near 2250 cm-1
Figure IR17. IR spectrum of benzonitrile. Source: SDBSWeb : http://riodb01.ibase.aist.go.jp/sdbs/ (National Institute of Advanced Industrial Science and Technology of Japan, 14 July 2008)
The combination of anisotropy and electronegativity causes the O-H hydrogen in a carboxylic acid to be very deshielded.
Hydrogen environments adjacent to a carboxylic acid are shifted to the region of 2.5-3.0 ppm.Deshielding occurs due to the fact that the sp2 hybridized carbon the the carboxylic acid is more electronegative than a sp3 hybridized carbon.



|
|
|
|
لخفض ضغط الدم.. دراسة تحدد "تمارين مهمة"
|
|
|
|
|
|
|
طال انتظارها.. ميزة جديدة من "واتساب" تعزز الخصوصية
|
|
|
|
|
|
|
مشاتل الكفيل تزيّن مجمّع أبي الفضل العبّاس (عليه السلام) بالورد استعدادًا لحفل التخرج المركزي
|
|
|