

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
The geometry and relative stability of carbon radicals
المؤلف:
..................
المصدر:
LibreTexts Project
الجزء والصفحة:
.................
25-7-2019
2074
The geometry and relative stability of carbon radicals
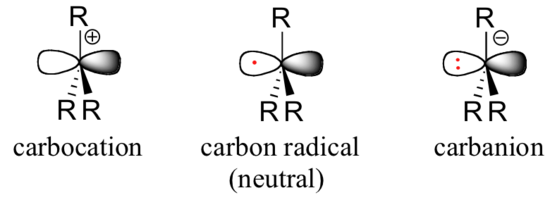
As organic chemists, we are particularly interested in radical intermediates in which the unpaired electron resides on a carbon atom. Experimental evidence indicates that the three bonds in a carbon radical have trigonal planar geometry, and therefore the carbon is considered to be sp2-hybridized with the unpaired electron occupying the perpendicular, unhybridized 2pzorbital. Contrast this picture with carbocation and carbanion intermediates, which are both also trigonal planar but whose 2pz orbitals contain zero or two electrons, respectively.
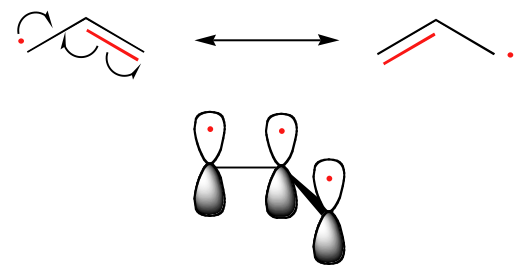
The trend in the stability of carbon radicals parallels that of carbocations: tertiary radicals, for example, are more stable than secondary radicals, followed by primary and methyl radicals. This should make intuitive sense, because radicals, like carbocations, can be considered to be electron deficient, and thus are stabilized by the electron-donating effects of nearby alkyl groups. Benzylic and allylic radicals are more stable than alkyl radicals due to resonance effects - an unpaired electron can be delocalized over a system of conjugated pi bonds. An allylic radical, for example, can be pictured as a system of three parallel 2pz orbitals sharing three electrons.
Electrons have no fixed position in atoms, compounds and molecules (see image below) but have probabilities of being found in certain spaces (orbitals). Resonance forms illustrate areas of higher probabilities (electron densities). This is like holding your hat in either your right hand or your left. The term Resonance is applied when there are two or more possibilities available. Resonance structures do not change the relative positions of the atoms like your arms in the metaphor. The skeleton of the Lewis Structure remains the same, only the electron locations change. A double headed arrow on both ends of the arrow ( ↔) between Lewis structures is used to show their inter-connectivity. It is different from the double harpoons ( ⇌ ) used for designating equilibria. A double headed arrow on only one end ( →) is used to indicate the movement of two electrons in a single resonance structure.
Example .1
Consider ozone (O3)
SOLUTION
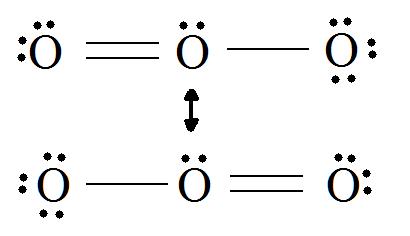
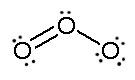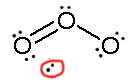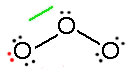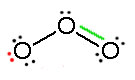
Figure: This is an animation of how one can do a resonance with ozone by moving electrons:
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















