
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 25-1-2019
Date: 14-12-2018
Date: 31-12-2018
|
Let's look at the first stage of the reduction - from VO2+ to VO2+. The redox potential for the vanadium half-reaction is given by:
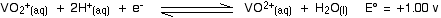
The corresponding equilibrium for the zinc is:
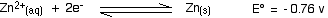
The simple principle is that if you couple two of these half-reactions together, the one with the more positive E° value will move to the right; the one with the more negative (or less positive) E° moves to the left. If you mix together zinc and VO2+ ions in the presence of acid to provide the H+ ions:
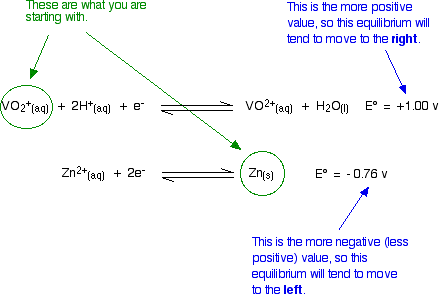
That converts the two equilibria into two one-way reactions. You can write these down and combine them to give the ionic equation for the reaction if you want to.
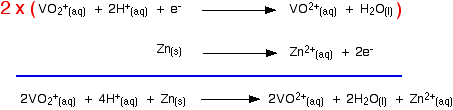



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|