
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 17-12-2020
Date: 18-12-2020
Date: 28-9-2018
|
Graphical method for determine the order of a reaction
For reactions of the type A →products, we can determine the reaction order by seeing whether a graph of the data fits one of the integrated rate equations.
In case of First order
We have already derived the integrated rate equation for first order as
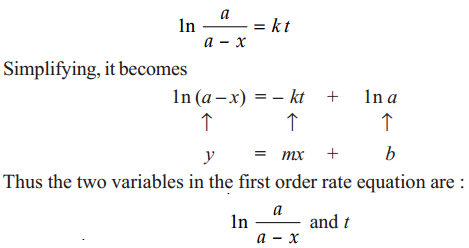
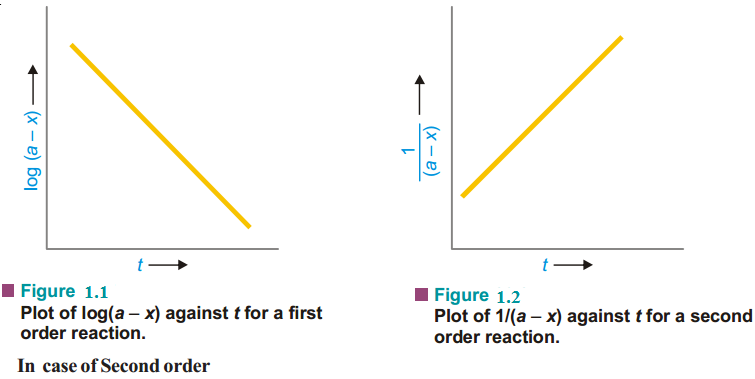
Hence, if 1n a/a–x is plotted against tand straight line results (Fig. 1.1), the corresponding reaction is of the first order. However, if a curve is obtained, the reaction is not first order.
We have already shown that second order rate equation can be written as
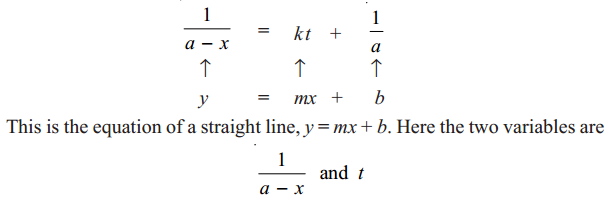
Thus when 1 / a−x is plotted against t and we get a straight line (Fig. 1.2), the reaction is second order. In case a curve is obtained, the reaction is not second order



|
|
|
|
دراسة: حفنة من الجوز يوميا تحميك من سرطان القولون
|
|
|
|
|
|
|
تنشيط أول مفاعل ملح منصهر يستعمل الثوريوم في العالم.. سباق "الأرنب والسلحفاة"
|
|
|
|
|
|
|
لتعزيز التواصل مع الزائرات الأجنبيات : العتبة العلويّة المقدّسة تُطلق دورة لتعليم اللغة الإنجليزية لخادمات القسم النسويّ
|
|
|