


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 3-8-2019
Date: 8-7-2019
Date: 21-7-2019
|
Cross-Conjugated Derivatives
Derivatives of 2,5-cyclohexadienone are common in nature, and their photochemical transformations posed a challenge to early researchers. Some reactions of 4,4-diphenyl-2,5-cyclohexadienone are presented in the following diagram. Although 2,3-diphenylphenol was a product from irradiation in aqueous dioxane, it is actually formed from 6,6-diphenylbicyclo[3.1.0]hex-3-ene-2-one, the initial rearrangement product. It should be noted that a similar bicyclo[3.1.0]hexane isomer was formed by irradiation of 4,4-diphenyl-2-cyclohexenones, as shown in the gray-shaded box at the bottom right.
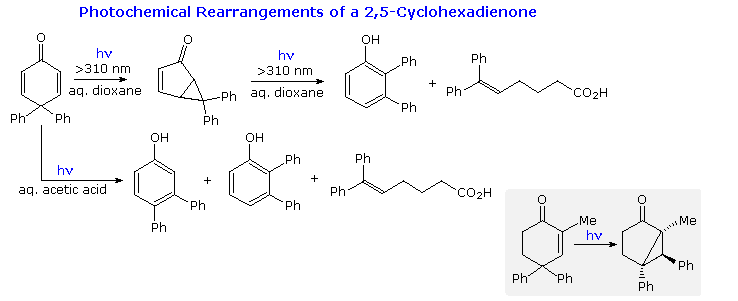
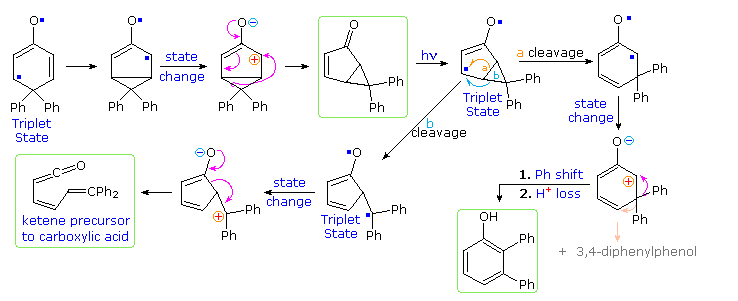
These photochemical rearrangements occur by way of triplet excited states, which are conveniently depicted as diradicals. Mechanisms for these reactions will be displayed above . Bond reorganization may take place in the triplets, which eventually intersystem cross to singlet species. Charge separation in these states may then lead to rearrangement to a stable product.
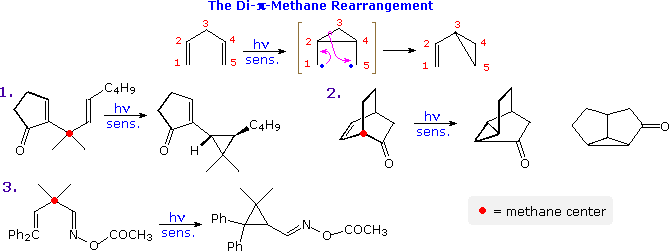
The photo-isomerization shown above is one example of a general family of reactions known as di-π-methane rearrangements, other examples of which are illustrated in the following diagram. These transformations are often photo-sensitized, indicating they proceed by way of triplet excited states. As the name suggests, substrates exhibiting this rearrangement are comprised of two π-functions separated by a saturated (sp3-hybrid) carbon atom (designated by a red dot in the illustration). In the event, a 1,2-shift of one ene-group, followed by a bond formation between the terminal sites of the remaining π-function leads to a vinylcyclopropane product. One of the unsaturated functions may be a carbonyl or imine group, as in examples 2. and 3.
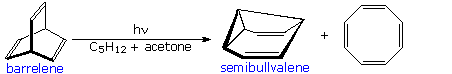
A photo-isomerization of this kind was used by H. Zimmerman (Wisconsin) to prepare the novel self-replicating diene semibullvalene from barrelene.



|
|
|
|
لخفض ضغط الدم.. دراسة تحدد "تمارين مهمة"
|
|
|
|
|
|
|
طال انتظارها.. ميزة جديدة من "واتساب" تعزز الخصوصية
|
|
|
|
|
|
|
مشاتل الكفيل تزيّن مجمّع أبي الفضل العبّاس (عليه السلام) بالورد استعدادًا لحفل التخرج المركزي
|
|
|