

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Substituent Effects on the Acidity of Carboxylic Acids
المؤلف:
William Reusch
المصدر:
Virtual Textbook of Organic Chemistry
الجزء والصفحة:
............
12-7-2018
2815
Substituent Effects on the Acidity of Carboxylic Acids
The carboxylic acids are a large and structurally diverse class of compounds. Since most are at least partially soluble in water and have pKa's in the 2 to 5 region, the influence of functional substituents and structural features on aqueous acidity have been studied extensively. Formic acid, HCO2H, is the simplest member of this class, and will serve as a useful reference point, pKa=3.75. Although the greater acidity of formic acid compared with methanol has been attributed to resonance stabilization of the formate anion, the different solvation demands of the respective conjugate anions result in an entropy difference that also favors the formate base. Both factors are depicted in the following illustration.. Resonance delocalization of the negative charge in the formate anion produces a large enthalpic stabilization shown by the magenta arrow. In water solution both methanol and formic acid are incorporated into the dynamic hydrogen bonded structure of liquid water. On ionization, each of these solutes produces a hydrated proton (hydronium ion) and a negatively charged conjugate base. The hydronium ion is common to both cases and can be ignored. The negative charge in the methoxide anion is concentrated on a single oxygen atom and demands strong solvation by water molecules, indicated by the aqua-colored dots. This solvation forces significant structural organization on many water molecules at the cost of decreased entropy. The formate anion also carries a single negative charge, but it is distributed over two oxygen atoms, so the charge density at either site is halved, compared with methoxide. This lower charge density demands much less solvation by water, resulting in a smaller entropy cost.
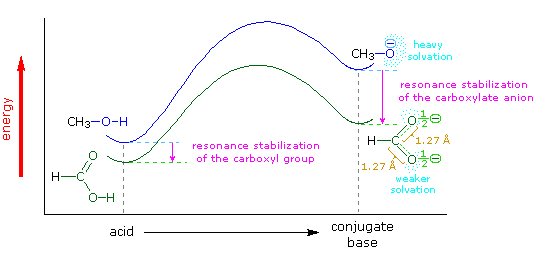
The importance of solvation and the accompanying entropy changes to any discussion of acidity may be seen by comparing the pKa's of methanol and formic acid in water and DMSO, a solvent that poorly solvates anions. In water the pKa of methanol is 15.5, nearly 12 powers of ten less acidic than formic acid (3.75). In DMSO the pKa's of methanol and formic acid are roughly 29 and 13 respectively, representing a very large decrease in Brønsted acid strength for both compounds (more than ten powers of ten). Furthermore, the difference in acid strength between methanol and formic acid in DMSO is magnified about ten thousand times, even though the enthalpic resonance stabilization presumably remains constant. A more extensive discussion of solvent effects on acidity was presented earlier. When comparing the acidities of different acids, care must be taken to use pKa's measured in the same solvent. In this discussion all the pKa's were taken in or extrapolated to water at 25 ºC. Measurements in mixed aqueous solvents, using water-soluble organic co-solvents such as ethanol, acetonitrile, dioxane, DMSO and acetone, generally give significantly larger pKa's.
In all other carboxylic acids an organic substituent replaces the hydrogen of formic acid, and it is instructive to analyze the change in acid strength caused by this change. To begin with, we must recognize that the carbonyl moiety of the carboxyl group is electrophilic and withdraws electrons from substituents. The deactivating nature of the carboxyl group on electrophilic substitution of benzoic acid is one example of this property. Resonance structures, such as A, B & C in the following diagram, are often drawn to describe this electrophilic character. The inductive effect of substituent Z in this diagram may enhance or diminish this character, depending on its overall electronegativity. Inductive electron withdrawal will increase the electrophilic character and the acidity of the carboxyl group, as shown in the green shaded box on the right. Resonance electron donation, either by p-π or π-π interaction, would act to stabilize the carboxylic acid, reducing its electrophilicity and acidity. These two effects often act in opposition, and in the case of carbonic acid ( H2CO3 ) electron donation overcomes inductive withdrawal, resulting in a pKa1=6.63.
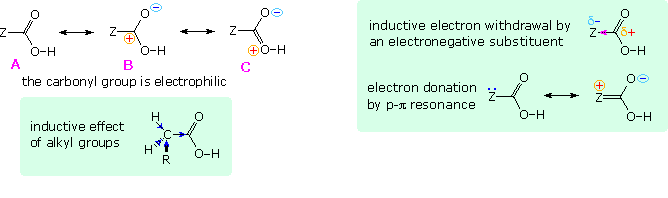
Saturated aliphatic acids are generally ten times weaker than formic acid, which may seem surprising since carbon has a higher Pauling electronegativity than hydrogen (2.55 versus 2.20). However, we must recognize that a carbon atom is larger and more polarizable than hydrogen, allowing it to shift electrons toward the more electronegative carbonyl carbon of the carboxyl group. Also, hydrogen and alkyl substituents on the α-carbon assist in this inductive electron shift, as shown in the green box on the left. This analysis is supported by the activating influence of alkyl substituents in electrophilic aromatic substitution, the Markovnikov rule, and the greater reactivity of aldehydes with nucleophiles compared with equivalent methyl ketones.
The four carboxylic acids in the first row of the following table illustrate the electron donating quality of alkyl groups. As the number of carbon atoms in the group increases from one to five, the inductive electron donation also increases. The compounds in the next three rows of the table demonstrate that electronegative substituents on an alkyl group can shift its inductive effect from donating to withdrawing (relative to hydrogen). Thus, all the haloacetic acids are more acidic than formic acid, with fluoroacetic acid being the most acidic. Additional halogen substituents have an additive influence, and moving the substituent from the α to a β-carbon reduces its influence on the acidity. Note that a hydroxyl substituent has a much weaker effect than any of the halogens, despite the higher electronegativity of oxygen (3.44 compared with 3.16 for chlorine).
|
pKa Values for Some Aliphatic Carboxylic Acids ( 25 ºC in H2O ) |
||||||||||
|
Compound |
pKa |
Compound |
pKa |
Compound |
pKa |
Compound |
pKa |
|||
|
CH3CO2H |
4.76 |
CH3CH2CO2H |
4.87 |
CH3(CH2)2CO2H |
4.91 |
(CH3)3CCO2H |
5.05 |
|||
|
FCH2CO2H |
2.59 |
ClCH2CO2H |
2.85 |
BrCH2CO2H |
2.89 |
ICH2CO2H |
3.13 |
|||
|
NCCH2CO2H |
2.50 |
HOCH2CO2H |
3.82 |
Cl2CHCO2H |
1.25 |
Cl3CCO2H |
0.77 |
|||
|
NCCH2CH2CO2H |
3.98 |
ClCH2CH2CO2H |
3.95 |
BrCH2CH2CO2H |
4.00 |
ICH2CH2CO2H |
4.06 |
|||
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















