
Adiabatic Atmosphere
 المؤلف:
Sidney B. Cahn, Gerald D. Mahan And Boris E. Nadgorny
المؤلف:
Sidney B. Cahn, Gerald D. Mahan And Boris E. Nadgorny
 المصدر:
A GUIDE TO PHYSICS PROBLEMS
المصدر:
A GUIDE TO PHYSICS PROBLEMS
 الجزء والصفحة:
part 2 , p 13
الجزء والصفحة:
part 2 , p 13
 25-8-2016
25-8-2016
 1495
1495
Adiabatic Atmosphere
The lower 10–15 km of the atmosphere, the troposphere, is often in a convective steady state with constant entropy, not constant temperature (PVγ is independent of the altitude, where γ = CP/CV).
a) Find the change of temperature in this model with altitude dT/dz.
b) Estimate dT/dz in K/km. Consider the average diatomic molecule of air with molar mass μ = 29 g/mole.
SOLUTION
a) Starting from the ideal gas law, we can express the temperature T as a function of pressure P and the mass density ρ:
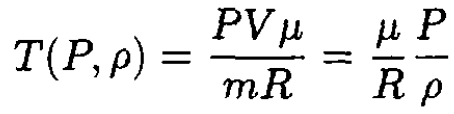 (1)
(1)
where P and ρ are functions of the height z above the surface of the Earth: P ≡ P(z), P ≡ ρ(z). Taking the derivative of T with respect to z, we have
 (2)
(2)
We need to express dρ in terms of dP. The fact that PVγ is independent of altitude allows us to write
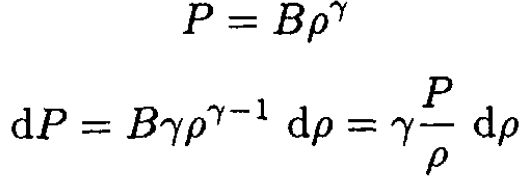
where B is some constant. So
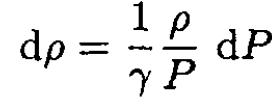 (3)
(3)
Substituting (3) into (2), we obtain
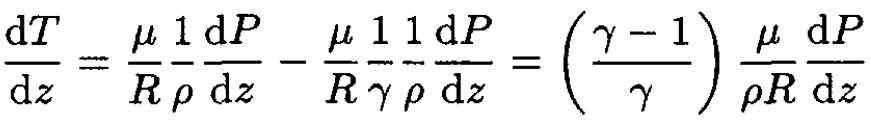 (4)
(4)
Assuming that the acceleration of gravity is constant, using the hydrostatic pressure formula
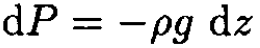 (5)
(5)
and substituting (5) into (4), we can write
 (6)
(6)
b) For the atmosphere, using diatomic molecules with CV = 5R/2 and CP = 7R/2, we have from (6),

This value of |dT/dz| is about a factor of 2 larger than that for the actual atmosphere.
 الاكثر قراءة في مواضيع اخرى
الاكثر قراءة في مواضيع اخرى
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


