
Molar Analytical Concentration
 المؤلف:
D. A. Skoog, F. J.Holler, D M. West, and S. R. Crouch
المؤلف:
D. A. Skoog, F. J.Holler, D M. West, and S. R. Crouch
 المصدر:
Fundamentals of Analytical Chemistry
المصدر:
Fundamentals of Analytical Chemistry
 الجزء والصفحة:
9th ed - p68
الجزء والصفحة:
9th ed - p68
 8-8-2016
8-8-2016
 3909
3909
Molar Analytical Concentration
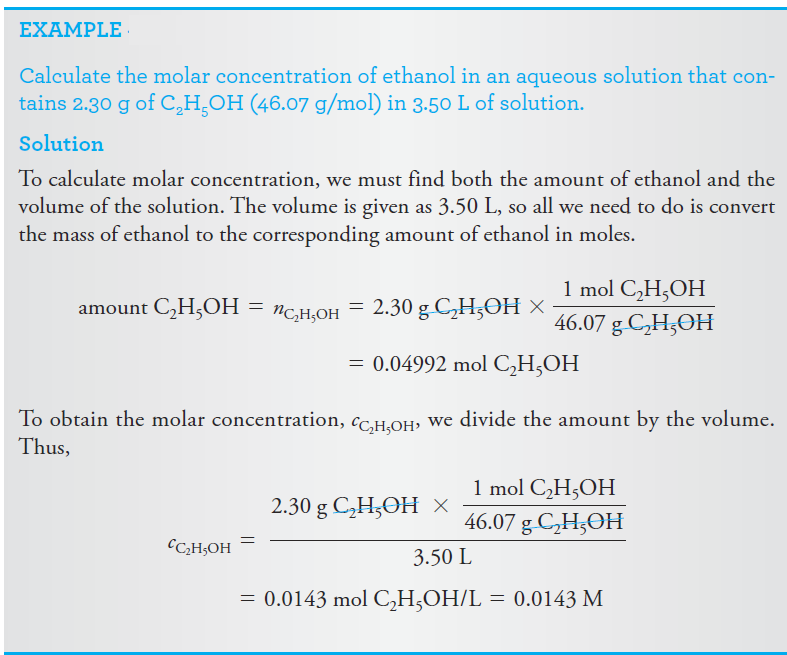
The molar analytical concentration, or for the sake of brevity, just analytical concentration, of a solution gives the total number of moles of a solute in 1 liter of the solution (or the total number of millimoles in 1 mL). In other words, the molar analytical concentration specifies a recipe by which the solution can be prepared regardless of what might happen to the solute during the solution process. Note that in Example below, the molar concentration that we calculated is also the molar analytical concentration c C2H5OH = 0.0143 M because the solute ethanol molecules are intact following the solution process.
In another example, a sulfuric acid solution that has an analytical concentration of c H2SO4 = 1.0 M can be prepared by dissolving 1.0 mole, or 98 g, of H2SO4 in water and diluting the acid to exactly 1.0 L. As we shall see, there are important differences between the ethanol and sulfuric acid examples.
 الاكثر قراءة في التحليل النوعي والكمي
الاكثر قراءة في التحليل النوعي والكمي
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


