

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Organic Acids and Organic Bases
المؤلف:
John McMurry
المصدر:
Organic Chemistry
الجزء والصفحة:
p47
10-3-2016
4047
Organic Acids and Organic Bases
Many of the reactions we’ll be seeing in future chapters, including practically all biological reactions, involve organic acids and organic bases. Although it’s too early to go into the details of these processes now, you might keep the following generalities in mind:
Organic Acids
Organic acids are characterized by the presence of a positively polarized hydrogen atom (blue in electrostatic potential maps) and are of two main kinds: acids such as methanol and acetic acid that contain a hydrogen atom bonded to an electronegative oxygen atom (O -H) and those such as acetonethat contain a hydrogen atom bonded to a carbon atom next to a C=O bond (O=C - C - H).
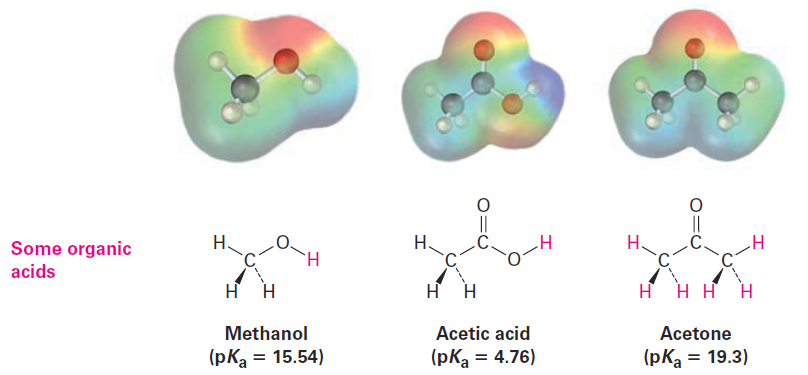
Methanol contains an O-H bond and is a weak acid, while acetic acid also contains an O-H bond and is a somewhat stronger acid. In both cases, acidity is due to the fact that the conjugate base resulting from loss of H+ is stabilized by having its negative charge on a strongly electronegative oxygen atom. In addition, the conjugate base of acetic acid is stabilized by resonance.
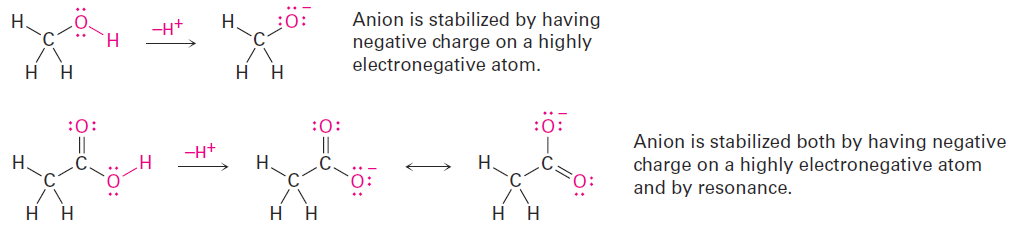
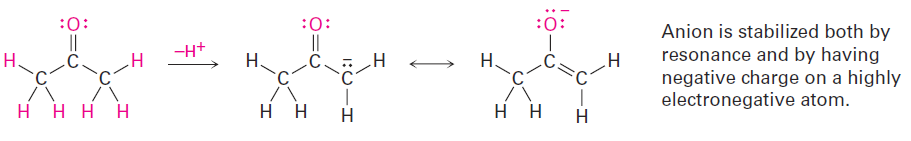
The acidity of acetone and other compounds with C=O bonds is due to the fact that the conjugate base resulting from loss of H+ is stabilized by resonance.
In addition, one of the resonance forms stabilizes the negative charge by placing it on an electronegative oxygen atom.
Electrostatic potential maps of the conjugate bases from methanol, acetic acid, and acetone are shown in Figure1. As you might expect, all three show a substantial amount of negative charge (red) on oxygen.
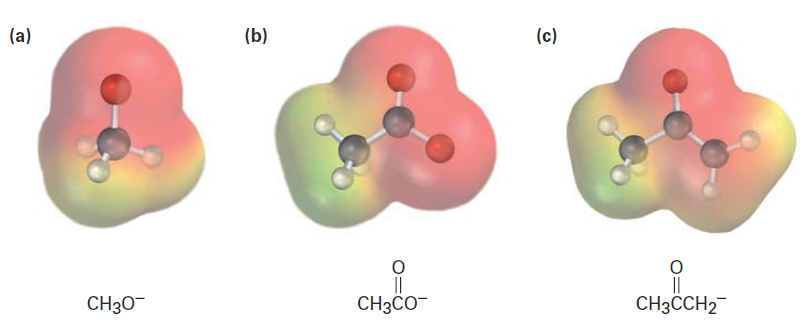
Figure.1 Electrostatic potential maps of the conjugate bases of (a) methanol, (b) acetic acid, and (c) acetone. The electronegative oxygen atoms stabilize the negative charge in all three.
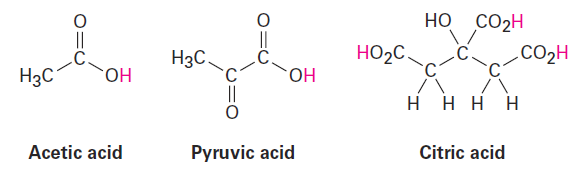
Compounds called carboxylic acids, which contain the -CO2H grouping, occur abundantly in all living organisms and are involved in almost all metabolic pathways. Acetic acid, pyruvic acid, and citric acid are examples. You might note that at the typical pH of 7.3 found within cells, carboxylic acids are usually dissociated and exist as their carboxylate anions, -CO2-.
Organic Bases
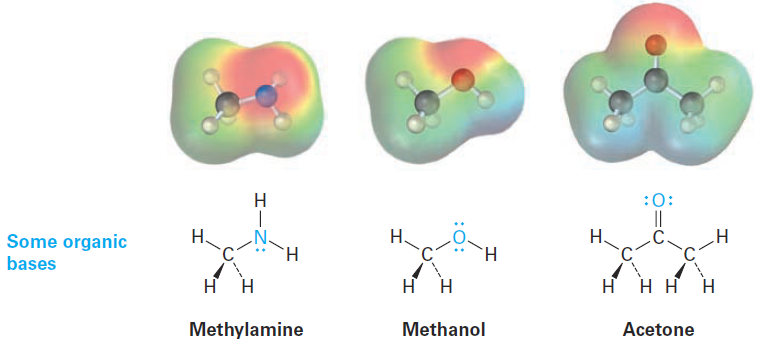
Organic bases are characterized by the presence of an atom (reddish in electrostatic potential maps) with a lone pair of electrons that can bond to H+.
Nitrogen-containing compounds such as methylamine are the most common organic bases and are involved in almost all metabolic pathways, but oxygencontaining compounds can also act as bases when reacting with a sufficiently strong acid. Note that some oxygen-containing compounds can act both as acids and as bases depending on the circumstances, just as water can. Methanol and acetone, for instance, act as acids when they donate a proton but as bases when their oxygen atom accepts a proton.
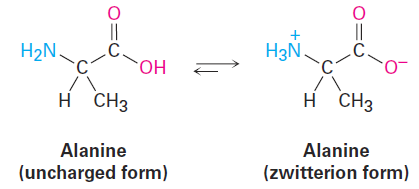
The substances that called amino acids, so-named because they are both amines ( -NH2) and carboxylic acids ( -CO2H), are the building blocks from which the proteins in all living organisms are made. Twenty different amino acids go into making up proteins—alanine is an example. Interestingly, alanine and other amino acids exist primarily in a doubly charged form called a zwitterion rather than in the uncharged form. The zwitterion form arises because amino acids have both acidic and basic sites within the same molecule and therefore undergo an internal acid–base reaction.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















