

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Describing Chemical Bonds: Molecular Orbital Theory
المؤلف:
John McMurry
المصدر:
Organic Chemistry
الجزء والصفحة:
9Th. p21
7-2-2016
3385
Describing Chemical Bonds: Molecular Orbital Theory
We said befor that chemists use two models for describing covalent bonds: valence bond theory and molecular orbital theory. Having now seen the valence bond approach, which uses hybrid atomic orbitals to account for geometry and assumes the overlap of atomic orbitals to account for electron sharing, let’s look briefly at the molecular orbital approach to bonding.
Molecular orbital (MO) theory describes covalent bond formation as arising from a mathematical combination of atomic orbitals (wave functions) on different atoms to form molecular orbitals, so called because they belong to the entire molecule rather than to an individual atom. Just as an atomic orbital, whether unhybridized or hybridized, describes a region of space around an atom where an electron is likely to be found, so a molecular orbital describes a region of space in a molecule where electrons are most likely to be found.
Like an atomic orbital, a molecular orbital has a specific size, shape, and energy. In the H2 molecule, for example, two singly occupied 1s atomic orbitals combine to form two molecular orbitals. There are two ways for the orbital combination to occur—an additive way and a subtractive way. The additive combination leads to the formation of a molecular orbital that is lower in energy and roughly egg-shaped, while the subtractive combination leads to a molecular orbital that is higher in energy and has a node between nuclei (Figure 1). Note that the additive combination is a single, egg-shaped, molecular orbital; it is not the same as the two overlapping 1s atomic orbitals of the valence bond description. Similarly, the subtractive combination is a single molecular orbital with the shape of an elongated dumbbell. The additive combination is lower in energy than the two hydrogen 1s atomic orbitals and is called a bonding MO because electrons in this MO spend most of their time in the region between the two nuclei, thereby bonding the atoms together. The subtractive combination is higher in energy than the two hydrogen 1s orbitals and is called an antibonding MO because any electrons it contains can’t occupy the central region between the nuclei, where there is a node, and can’t contribute to bonding.
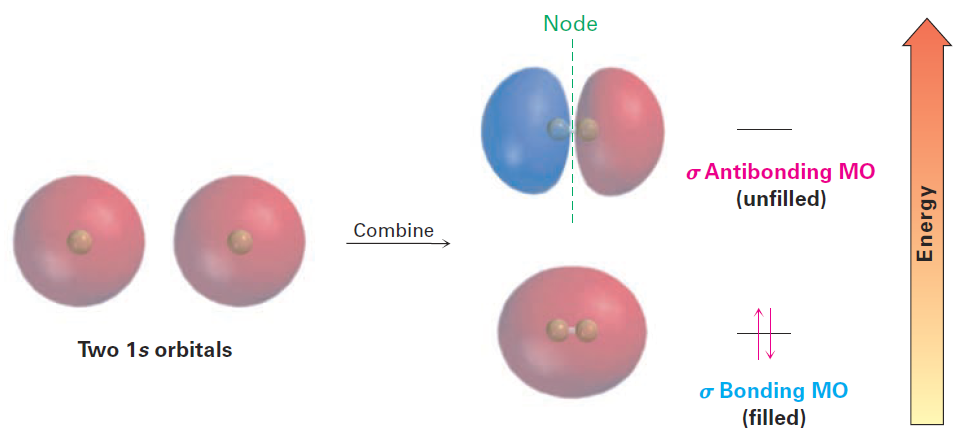
Figure 1 . Molecular orbitals of H2. Combination of two hydrogen 1s atomic orbitals leads to two H2 molecular orbitals. The lower-energy, bonding MO is filled, and the higher-energy, antibonding MO is unfilled.
Just as bonding and antibonding σ molecular orbitals result from the headon combination of two s atomic orbitals in H2, so bonding and antibonding π molecular orbitals result from the sideways combination of two p atomic orbitals in ethylene. As shown in Figure 2. the lower-energy, π bonding MO has no node between nuclei and results from the combination of p orbital lobes with the same algebraic sign. The higher-energy, π antibonding MO has a node between nuclei and results from the combination of lobes with opposite algebraic signs. Only the bonding MO is occupied; the higher-energy, antibonding MO is vacant.
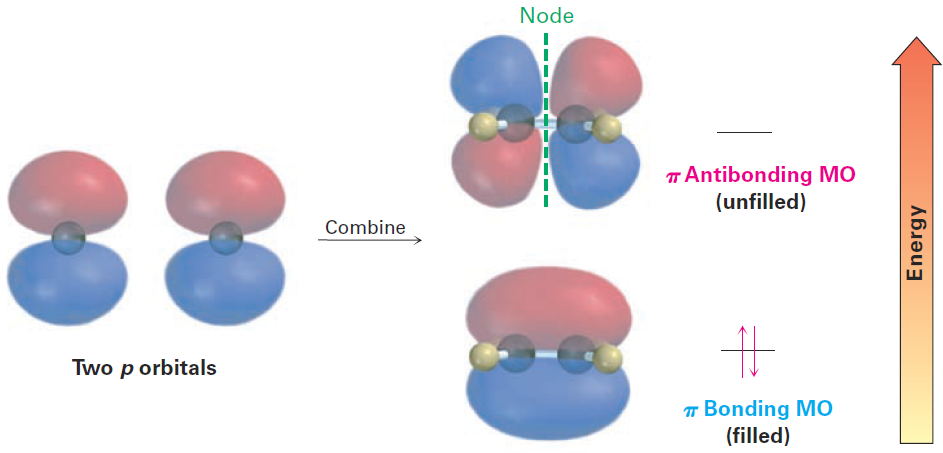
Figure 2. A molecular orbital description of the C—C π bond in ethylene.
The lower-energy, π bonding MO results from an additive combination of p orbital lobes with the same algebraic sign and is filled. The higherenergy, π antibonding MO results from a subtractive combination of p orbital lobes with opposite algebraic signs and is unfilled.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















