
Special ligands
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
729
الجزء والصفحة:
729
 2025-10-22
2025-10-22
 290
290
Special ligands
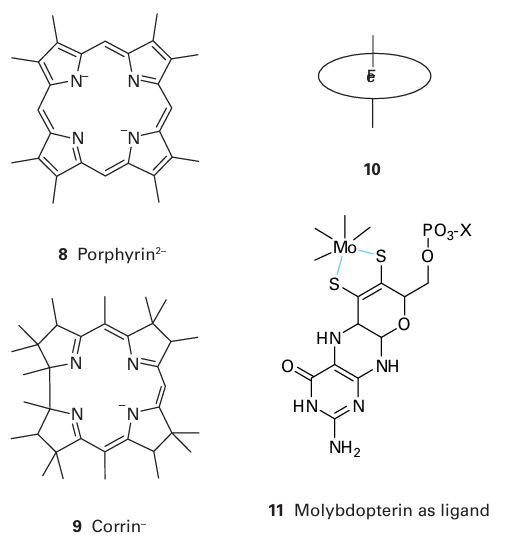
Key point: Metal ions may be bound in proteins by special organic ligands such as porphyrins and pterin-dithiolenes. The porphyrin group (8) was first identified in haemoglobin (Fe) and a similar macrocycle is found in chlorophyll (Mg). There are several classes of this hydrophobic macrocycle, each differing in the nature of the side chains. The corrin ligand (9) has a slightly smaller ring size and coordinates Co in cobalamin (Section 27.11). Rather than show these macro cycles in full, we shall use shorthand symbols such as (10) to show the complexes they form with metals. Almost all Mo and W enzymes have the metal coordinated by a special ligand known as molybdopterin (11). The donors to the metal are a pair of S atoms from a dithiolene group that is covalently attached to a pterin. The phosphate group is often joined to a nucleoside base X, such as guanosine 5-phosphate (GMP), resulting in the formation of a diphosphate bond. Why Mo and W are coordinated by this complex ligand is unknown, but the pterin group could provide a good electron conduit and facilitate redox reactions.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


