
Chemisorption and desorption
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص707-708
الجزء والصفحة:
ص707-708
 2025-10-19
2025-10-19
 360
360
Chemisorption and desorption
Key point: Adsorption is essential for heterogeneous catalysis to occur but must not be so strong that it blocks the catalytic sites and prevents further reaction. The adsorption of molecules on surfaces often activates molecules just as coordination activates molecules in complexes. The desorption of product molecules that is necessary to refresh the active sites in heterogeneous catalysis is analogous to the dissociation of a complex in homogeneous catalysis. Before a heterogeneous catalyst is used it is usually ‘activated’. Activation is a catch-all term. In some instances it refers to the desorption of adsorbed molecules such as water from the surface, as in the dehydration of γ-alumina. In other cases it refers to the preparation of the active site by a chemical reaction, such as by reduction of metal oxide particles to produce active metal particles. An activated surface can be characterized by the adsorption of various inert and re active gases. The adsorption may be either physisorption, when no new chemical bond is formed, or chemisorption, when surface–adsorbate bonds are formed (Fig. 26.16). Low temperature physisorption of a gas such as nitrogen is useful for the determination of the total surface area of a solid, whereas chemisorption is used to determine the number of exposed reactive sites. For example, the dissociative chemisorption of H2 on supported platinum particles reveals the number of exposed surface Pt atoms. The interaction of small molecules with metal surfaces is similar to their interaction with low-oxidation-state metal complexes. Table 26.4 shows that a wide range of metals chemisorb CO, and that fewer are capable of chemisorbing N2, just as there is a much wider variety of metals that form carbonyls than form dinitrogen complexes. Furthermore, just as with metal carbonyl complexes, both bridging and terminal CO surface species have been identified by IR spectroscopy. The dissociative chemisorption of H2 is analogous to the oxidative addition of H2 to metal complexes (Sections 10.5 and 22.7).
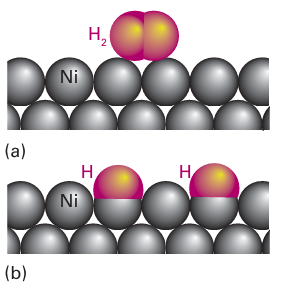
Figure 26.16 Schematic representation of (a) physisorption and (b) chemisorption of hydrogen and a nickel metal surface.
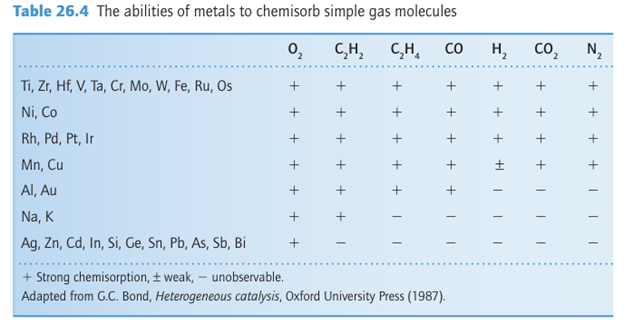
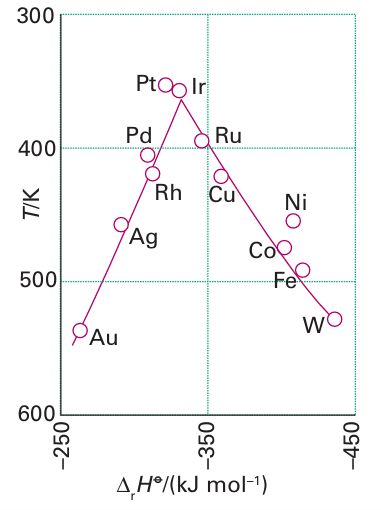
Figure 26.17 A volcano diagram. In this case the reaction temperature for a set rate of methanoic (formic) acid decomposition plotted against the stability of the corresponding metal methanoate as judged by its enthalpy of formation. (Based on W.J.M. Rootsaert and W.M.H. Sachtler, Z. Phyzik. Chem. 1960, 26 16.)
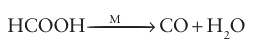
provides a good example of this balance between adsorption and catalytic activity. It is observed that the catalysis is most effective using metals for which the metal methanoate is of intermediate stability (Fig. 26.17). The plot in Fig. 26.17 is an example of a ‘volcano diagram’, and is typical of many catalytic reactions. The implication is that the earlier d-block metals form very stable surface compounds whereas the later noble metals such as silver and gold form very weak surface compounds, both of which are detrimental to a catalytic process. Between these extremes the metals in Groups 8 to 10 have high catalytic activity, especially the platinum metals (Group 10). In Section 26.4 we saw a simi lar high activity of these metal complexes in the homogeneous catalysis of hydrocarbon transformations. The active sites of heterogeneous catalysts are not uniform and many diverse sites are exposed on the surface of a poorly crystalline solid such as γ-alumina or a noncrystalline solid such as silica gel. However, even highly crystalline metal particles are not uniform. A crystalline solid has typically more than one type of exposed plane, each with its char acteristic pattern of surface atoms (Fig. 26.18). In addition, single-crystal metal surfaces have irregularities such as steps that expose metal atoms with low coordination numbers (Fig. 26.19). These highly exposed, coordinatively unsaturated sites appear to be particularly reactive. As a result, the different sites on the surface may serve different functions in catalytic reactions. The variety of sites also accounts for the lower selectivity of many heterogeneous catalysts in comparison with their homogeneous analogues.
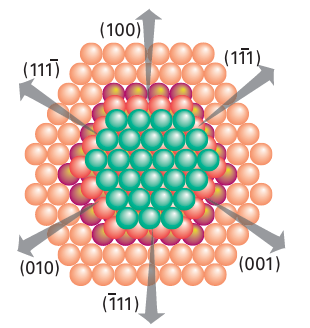
Figure 26.18 Some possible metal crystal planes that might be exposed on a metal surface to a reactive gas. The planes labelled [1–11], [11–1], etc. are hexagonally close- packed and the planes represented by [100], [010], etc. have square arrays of atoms.
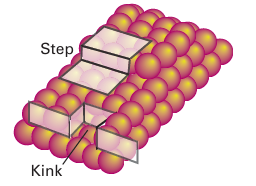
Figure 26.19 Schematic representation of surface irregularities, steps, and kinks.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


