
Nanosized reaction vessels
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص662-664
الجزء والصفحة:
ص662-664
 2025-10-13
2025-10-13
 306
306
Nanosized reaction vessels
Key points: By carrying out reactions in nanoscale reaction vessels, the ultimate dimensions of solid products are confined to the vessel size; a reverse micelle has an aqueous core in which reactions can occur. When the synthesis of a particle is carried out in a nano-vessel, the ultimate particle size is limited by the vessel size. One popular route is the inverse micelle synthesis approach. An inverse micelle consists of a two-phase dispersion of immiscible liquids, such as water and a nonpolar oil. By including amphipathic surfactant molecules (molecules having a polar and a nonpolar end), the aqueous phase can be stabilized as dispersed spheres with a size dictated by the water: surfactant ratio (Fig. 25.7). The size of the crystalline particles is limited by the micelle volume, which can be controlled on the nanoscale. Examples of nanoparticles formed by this technique are Cu, Fe, Au, Co, CdS, CdSe, ZrO2, ferrites, and core–shell particles such as Fe/Au. An alternative approach to nanopatterning is laser-assisted embossing, which has been used to generate close-packed arrays of hemispherical nanowells having volumes of the order of zeptolitres (1 zL=10-21 L= 10-25 m3= 103 nm3). The volumes of the nanowells are manipulated by changing the intensity of the laser, by altering the pressure between the mould and the substrate, and by growing an amorphous oxide on the patterned silicon prior to nanocrystallization. Nanowells with diameters as small as 50 nm have been used as reaction chambers for the preparation of semiconducting nanocrystals. Under most growth conditions, independent nanocrystals are formed in individual nanowells (Fig. 25.8).
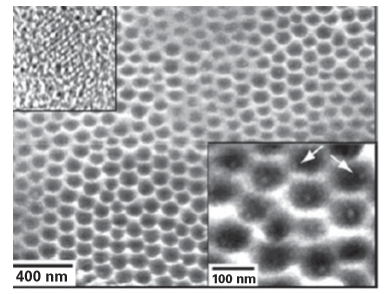
Figure 25.8 An SEM image of an array of nanoscale wells that contain one CdS nanocrystal per well. The lower inset magnifies a small area of the array; the white arrows indicate crystals formed at the edges of the wells. The upper inset is a TEM image of an individual CdS nanocrystal removed from the array of nanowells by sonication. (J.E. Barton and T.W. Odom, Nano Lett., 2004, 4, 1525.)

As discussed earlier, high-temperature (150–250ºC) solution synthesis is one of the most successful methods for achieving size and shape control of nanocrystalline materials through nucleation and growth in the presence of selective surfactant molecules, including TOPO. However, inhomogeneity in the injection of precursors, mixing of the reactants, and temperature gradients in the reaction flask can lead to problems with control over the size distribution of nanoparticles. Small reaction vessels, such as reverse micelles and laserembossed surfaces, provide an appealing alternative for controlling chemical and thermal homogeneity. The use of nanofabricated structures made from these materials enables the growth and assembly of nanocrystals that are potentially more interesting than inorganic salts and organic molecular crystals. This approach to the growth of nanocrystals has several advantages, including facile syntheses based on bulk reactions, flexible size control achieved by using different concentrations of precursor materials, realization of isolated nanocrystals in ordered arrays, and reusable templates.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


