
Magnesium-based metal hydrides
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
639
الجزء والصفحة:
639
 2025-10-13
2025-10-13
 232
232
Magnesium-based metal hydrides
Magnesium hydride, MgH2 (rutile structure type, Fig. 24.59) potentially offers a high capacity of 7.7 per cent hydrogen by mass combined with the low cost of magnesium and good reversibility for hydrogen uptake and evolution at high temperatures. However, its decomposition temperature of 300°C under 1 atm of hydrogen gas is uneconomically high and considerable effort has been expended to develop new related materials that have lower hydrogen release temperatures. Doping of MgH2 with a variety of metals has been undertaken to produce the solid solutions Mg1-xMxH2±y for M = Al, V, Ni, Co, Ti, Ge, and La/Ni mixtures, some of which have slightly lower decomposition temperatures. More promising are changes in the microstructure of MgH2 which are caused by ball-milling (where the solid is shaken vigorously with small hard spheres inside a canister), particularly when done in combination with other materials, such as metal (Ni, Pd) and metal oxides (V2O5, Cr2O3). Ball-milling of MgH2 increases the surface area of the solid by about a factor of 10 and the number of defects in the crystallites, promoting both the adsorption and deadsorption of hydrogen.
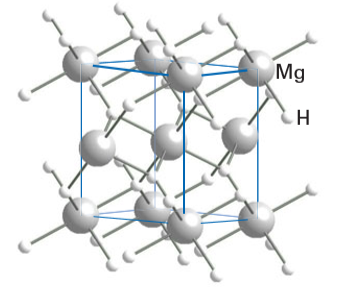
Figure 24.59 The structure of MgH2.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


