
Phosphates and silicates
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
635
الجزء والصفحة:
635
 2025-10-12
2025-10-12
 302
302
Phosphates and silicates
Key point: Calcium hydrogen phosphates are inorganic materials used in bone formation. Another tetrahedral oxoanion that is frequently incorporated into framework materials is the phosphate group, PO43-, although, as we shall see in the next section, many other tetrahedral units also form such structures. Simple phosphate structures are described in Section 15.15 and are generally formed from linked PO4 tetrahedra in chains, cross-linked chains, and cyclic units. We consider just one metal phosphate material in more detail here, namely calcium hydrogen phosphate, and closely related materials. The principal mineral present in bone and teeth is hydroxyapatite, Ca5(OH)(PO4)3, the structure of which consists of Ca2- ions coordinated by PO4 3- and OH groups to produce a rigid three-dimensional structure (Fig. 24.52). The mineral apatite is the partially fluoride-substituted Ca5(OH,F)(PO4)3. Related biominerals are Ca8H2(PO4)6 and amorphous forms of calcium phosphate itself
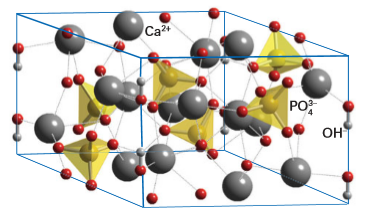
Figure 24.52 The structure of hydroxyapatite, Ca5(OH)(PO4)3, shown as Ca2ions coordinated by phosphate and OH ions into a strong three-dimensional structure. The separate O atoms are actually OH ions with the H lying outside the unit cell.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


