
Fluorides and other halides
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
626
الجزء والصفحة:
626
 2025-10-11
2025-10-11
 252
252
Fluorides and other halides
Key point: Because fluorine and oxygen have similar ionic radii, fluoride solid-state chemistry parallels much of oxide chemistry. The ionic radii of F− and O2− are very similar (at between 130 and 140 pm), and as a result metal fluorides show many stoichiometric and structural analogies with the complex oxides but with lower charge on the metal ion to reflect the lower charge on the F ion. Many binary metal fluorides adopt the simple structural types expected on the basis of the radius-ratio rule (Section 3.10). For example, FeF2 and PdF2 have a rutile structure and AgF has a rock-salt structure; similarly, NbF3 adopts the ReO3 structure. For complex fluorides, analogues of typical oxide structural types are well known, including perovskites (such as KMnF3), Ruddlesden–Popper phases (for exam ple K3 Co2F7), and spinels (Li2NiF4). Synthetic routes to complex fluorides also parallel those for oxides. For instance, the direct reaction of two metal fluorides yields the com plex fluoride, as in
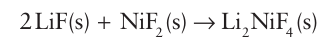
Like some complex oxides, some complex fluorides may be precipitated from solution
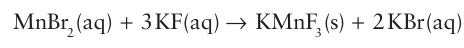
As with the ammonolysis of oxides to produce nitrides and oxide-nitrides, the formation of oxide-fluorides is possible by the appropriate treatment of a complex oxide, as in
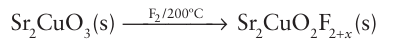
Sr2CuO2F2+x is a superconductor with Tc=45 K. Fluoride analogues of the silicate glasses, which are based on linked SiO4 tetrahedra, exist for small cations that form tetrahedral units in combination with F; an example is LiBF4, which contains linked BF4 tetrahedra. Lithium borofluoride glasses are used to contain samples for X-ray work because they are highly transparent to X-rays on account of their low electron densities. Framework and layer structures based on linked MF4 (M Li, Be) tetrahedra have also been described. Some metal fluorides are used as fluorination agents in organic chemistry. However, few of the solid complex metal fluorides are technologically important in comparison with the wealth of applications associated with analogous complex oxides. Metal chloride structures reflect the greater covalence associated with bonding to chloride in comparison with fluoride: the chlorides are less ionic and have structures with lower coordination numbers than the corresponding fluorides. Thus, simple metal chlorides, bromides, and iodides normally adopt the cadmium-chloride or cadmium-iodide structures based on sheets formed from edge-sharing MX6 octahedra. Complex chlorides often contain the same structural unit, for example CsNiCl3 has chains of edge-sharing NiCl6 octahedra separated by Cs ions. Many analogues of oxide structures also occur among the complex chlorides, such as KMnCl3, K2MnCl4, and Li2MnCl4, which have the perovskite, K2NiF4, and spinel structures, respectively .
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


