
Nitrides
 المؤلف:
المؤلف:
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص625-626
الجزء والصفحة:
ص625-626
 2025-10-11
2025-10-11
 274
274
Nitrides
Key points: Complex metal nitrides and oxide nitrides are materials containing the N3 anion; many new compounds of this type have recently been synthesized. Simple metal nitrides of main-group elements, such as AlN, GaN, and Li3N, have been known for decades. Many of the recent advances in nitride chemistry have centred on d-metal compounds and complex nitrides. That nitrides are less common than oxides stem, in part, from the high enthalpy of formation of N3 compared with that of O2. Further-more, because many nitrides are sensitive to oxygen and water, their synthesis and handling are problematic. Some simple metal nitrides can be obtained by the direct reaction of the elements, for example Li3N is obtained by heating lithium in a stream of nitrogen at 400ºC. The instability of sodium nitride allows sodium azide to be used as a nitriding agent:

The ammonolysis of oxides (the dehydrogenation of NH3 by oxides with the formation of water as a byproduct) provides a convenient route to some nitrides. For instance, tantalum nitride can be obtained by heating tantalum pentoxide in a fast-flowing stream of ammonia:
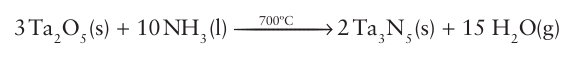
In such reactions, the equilibrium is driven towards the products by removal of the steam in the gas flow. Similar reactions may be used for the preparation of complex nitrides from complex oxides, although competing reactions involving partial reduction of the metal oxide by ammonia can also occur. In all these reactions the complete elimination of O2 ions from the product can be troublesome, so the reactions give products that contain both the oxide and nitride ions:

In comparison with the O2 ion, the higher charge of the N3 ion results in a greater degree of covalence in its bonding and therefore nitrides, particularly those of less electropositive elements such as d metals, should not be described in purely ionic terms. There is also a tendency with nitrides for the formation of compounds in which the metallic element is in a lower oxidation state because nitrogen, on account of its high bond energy, is not as potent an oxidant as oxygen or fluorine. Thus, whereas heating titanium in oxygen readily produces TiO2 , Ti2N and TiN are known but Ti3N4 is difficult to prepare and poorly characterized. Likewise, V3N5 is unknown whereas V2 O5 is readily obtained from the de composition of many vanadium salts in air. Many of the early d-metal nitrides are interstitial compounds and are used as high temperature refractory ceramics. Similarly, the nitrides of Si and Al, such as Si3 N4 (Fig. 24.35), are stable at very high temperatures, particularly under nonoxidizing
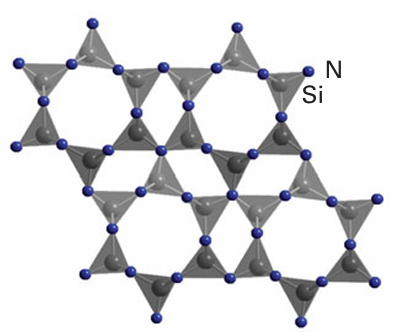
Figure 24.35 The structure of Si3N4 shown as linked SiN4 tetahedra. conditions, and are used for crucibles and furnace elements. Recently, GaN, which can exist in both wurtzite and sphalerite structural types, has been the focus of considerable research on account of its semiconducting properties. The nitride Li3N has an unusual structure based on hexagonal Li2N layers separated by Li ions (Fig. 24.8). These Lions are highly mobile, as is expected from the existence of free space between the layers, and this compound and other structurally related materials are being studied for possible use in rechargeable batteries. Among the many complex nitrides that have been synthesized are materials of stoichiometry AMN2, such as SrZrN2 and CaTaN2, with structures based on sheets formed from MN6 octahedra sharing edges (see the discussion of LiCoO2 in Section 24.7h) and A2MN3, such as Ba2VN3, containing one-dimensional chains of corner sharing MN4 tetrahedra (Fig. 24.36). Analogues of the zeolites containing nitride bridges rather than oxide bridges have also been prepared.

Figure 24.36 Ba2VN3, containing one dimensional chains of corner-sharing VN4 tetrahedra separated by Ba+2 ions.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


